Discussion
Rhabdomyolysis has been defined in various ways in previous studies. A CK level of at least 1,000 U/L or exceeding 5 times the upper limit of the normal range has been commonly used as the cutoff value for diagnosis [
4], but those of at least 5,000 U/L and at least 10,000 U/L have also been adopted [
8–
10]. Another criterion that is less frequently used is myoglobin, whose reference level in previous studies ranged from at least 80 ng/mL to at least 150 ng/mL [
7,
11,
12]. We used either CK or myoglobin level as the inclusion criteria for our study to maximize the number of participants.
Because of the diversity of definitions and subjects, the incidence of rhabdomyolysis varies from 4% to 50% in adult studies [
3,
11]. The exact incidence of pediatric rhabdomyolysis is unclear, but it has been reported to range from 5% to 37.5% [
5–
7]. Approximately 4% to 50% of cases of rhabdomyolysis lead to AKI, while up to 15% of AKI cases can be attributed to rhabdomyolysis [
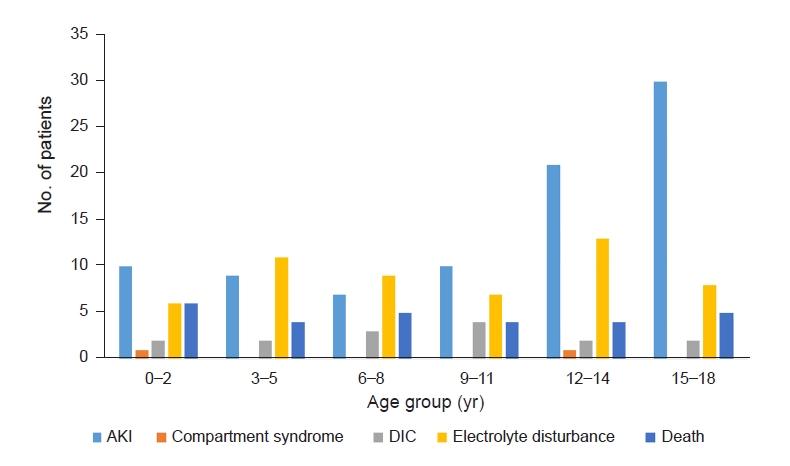
1–
3]. The incidence of AKI in our study was 11.3%. We believe that the use of a single, universal definition of rhabdomyolysis is necessary because it will aid in comparing incidence rates between studies and lead to advancements in this field.
A pediatric study performed in Korea investigated rhabdomyolysis in 39 children and reported the findings of a higher proportion of boys (2.5-fold more) among the affected children, epilepsy as the most common underlying disorder, and infection as the most common cause, similar to our results [
7]. Another pediatric study that involved 172 children in Taiwan reported the existence of a higher proportion of boys (3.7-fold more) among the total cases and found viral myositis to be the most common cause. However, these authors also reported an absence of sex differences in AKI incidence [
6]. Similarly, our study also revealed a male sex dominance. The higher proportion of adolescent males in rhabdomyolysis cases suggests that sex hormones or greater physical activity, which leads to more muscle mass, may contribute to the development of rhabdomyolysis. There were no sex differences in the proportion of causes, underlying disease, or complications of rhabdomyolysis in our study. Further studies are needed to determine whether sex contributes to rhabdomyolysis. We also did not observe any differences in the incidence of AKI according to sex. Therefore, male sex is not a risk factor for rhabdomyolysis-induced AKI.
The influence of anthropometric data on rhabdomyolysis and AKI has not been fully elucidated, and reports on the association between rhabdomyolysis and obesity are limited. Increased BMI could reflect a large muscle mass but usually implies excess adipose tissue that could induce pathological changes in the kidney and increase baseline circulating levels of nephrotoxic inflammatory molecules [
13]. Several adult studies have indicated that obesity and high BMI are linked to rhabdomyolysis and AKI [
14–
17]. In a single-center cohort study on obesity and AKI unrelated to rhabdomyolysis, obese patients were more likely to develop AKI, and each 5-kg/m
2 increase in BMI was associated with a 10% greater risk of severe AKI [
14]. However, BMI was not associated with AKI in a previous pediatric study [
6], and the z-scores of height, weight, and BMI did not differ between the AKI and non-AKI subgroups in our study. At this time, the reason why there is a contradiction regarding the influence of obesity on rhabdomyolysis and AKI between adults and children remains unclear. With respect to the different results between pediatric and adult studies, limitations of BMI, body composition, and duration of obesity might affect the risk of AKI.
Although myalgia, weakness, and dark-colored urine have been known as the classic triad of symptoms of rhabdomyolysis, these findings are observed in less than 10% of affected patients. In a previous report, more than 50% of patients did not have myalgia or weakness, while dark-colored urine was observed in only 3.6% of the cases [
2]. Our cases showed a relatively greater proportion of the classic triad than found in previous studies, but these symptoms were not distinguishing features of the AKI subgroup.
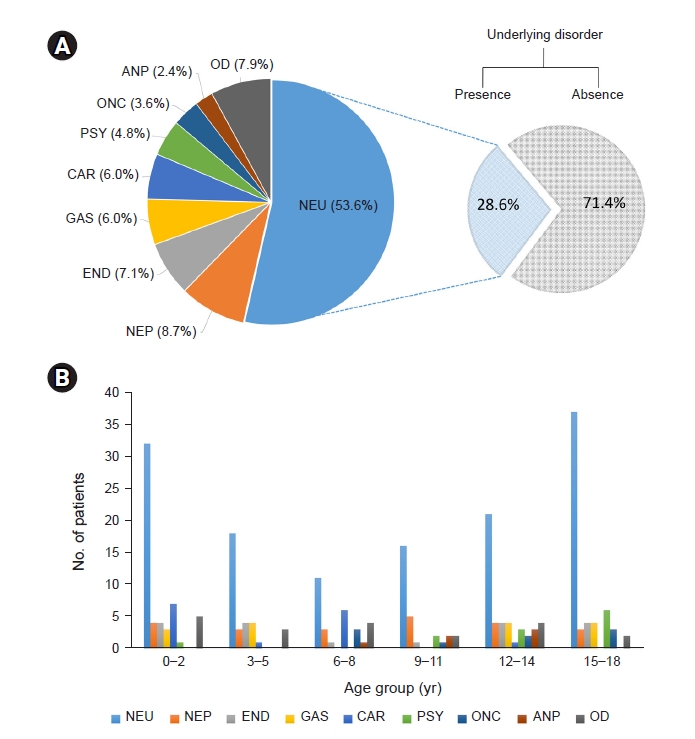
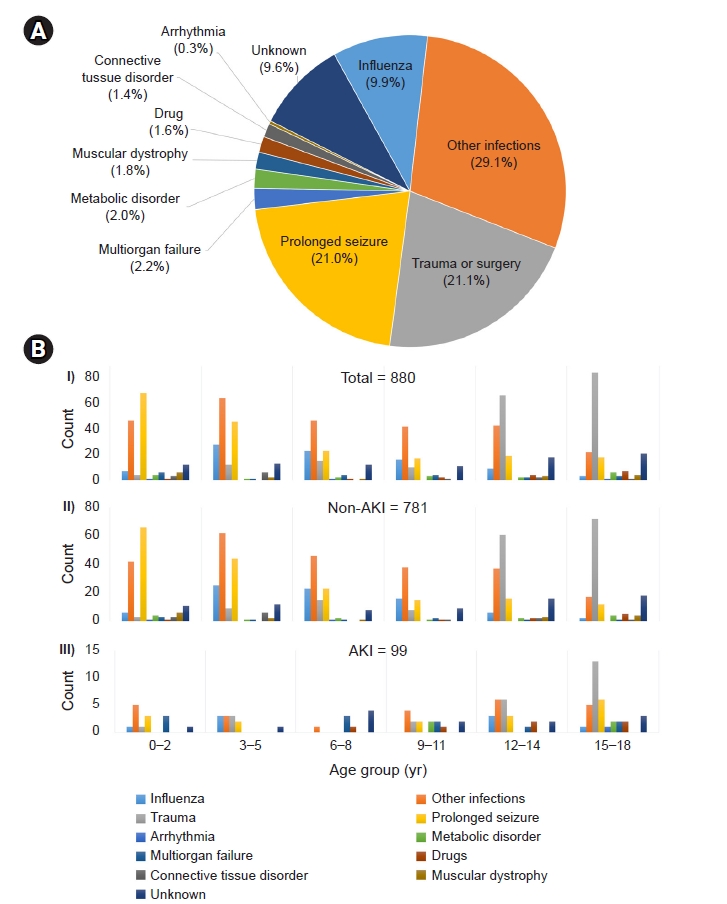
It is well known that infants and toddlers are vulnerable to febrile illness and seizures and that outside activities and accidents tend to increase in adolescents. The etiologic distribution of bimodal distribution in our study reflects these characteristics and may influence the frequency of rhabdomyolysis in each age group. Therefore, these etiological results vary depending upon the subpopulation. Previous adult studies suggested that trauma and illicit drug abuse are primary causes of rhabdomyolysis [
18–
20]. In our study, trauma was the second most common cause of rhabdomyolysis and was more prevalent in adolescent patients. However, drug-induced rhabdomyolysis accounted for only 1.7% of cases, and there were no cases of illicit drug use. Relatively low frequencies of trauma-induced and drug-induced rhabdomyolysis are believed to reflect the characteristics of the pediatric population and low prevalence of drug abuse in Korea [
21,
22]. Therefore, we suggest that the causes of rhabdomyolysis should be investigated in specific subpopulations.
Patients who experience seizures are considered vulnerable to rhabdomyolysis for two reasons. First, seizures lead to postictal alterations in several blood parameters, such as CK [
23]. It is well known that skeletal muscle injury can be caused by convulsive seizures or status epilepticus [
24]. Second, the majority of antiepileptic drugs, such as valproic acid and levetiracetam, are relevant to rhabdomyolysis [
25]. Some neuromuscular disorders, such as muscular dystrophy, are accompanied by increased CK levels, which are susceptible to triggering factors such as fever, exercise, bisphosphonate use, and anesthesia [
26–
28]. A retrospective study reported 13 patients with rhabdomyolysis featuring muscular dystrophy; the median duration between the first episodes of rhabdomyolysis and genetic diagnosis was 2 years, and the authors suggested that persistently increased CK levels with recurrent rhabdomyolysis warrant a workup for underlying muscular dystrophy [
28].
Muscle-derived components are effective biomarkers for renal damage, resulting in hyperphosphatemia; hypocalcemia; hyperuricemia; increased plasma LDH and AST levels; and increased urinary excretion of creatinine, uric acid, and glucose [
29]. Although CK is a cornerstone of rhabdomyolysis diagnosis, it remains controversial whether CK itself is related to the risk of AKI and mortality. Some studies have shown that the initial or peak CK value is not a reliable marker of AKI or mortality outcome [
30–
32]. However, other studies have suggested that the initial and peak values of CK and myoglobin are risk factors for AKI [
6,
20]. Our study showed that the initial CK level was significantly increased, but that of myoglobin was not. These contradictory results regarding CK and myoglobin may be affected by differences in the time interval from symptom onset to arrival at the hospital. CK levels rise in rhabdomyolysis within 12 hours of the onset of muscle injury, peak within 24 to 72 hours, and normalize approximately 5 days after the cessation of muscle injury. The half-life of CK is approximately 36 hours [
33]. Meanwhile, myoglobin has a short half-life (2–4 hours) and may return to normal within 6 to 8 hours [
2]. In future studies, it is worth considering adjusting the initial CK level according to the timing of muscle injury.
Our study revealed significant differences in most traditional biomarkers, including AST, ALT, LDH, uric acid, calcium, and phosphorus. Although they were not included in our study, constant albuminuria and hypoalbuminemia have been suggested as independent risk factors for AKI in previous research [
7,
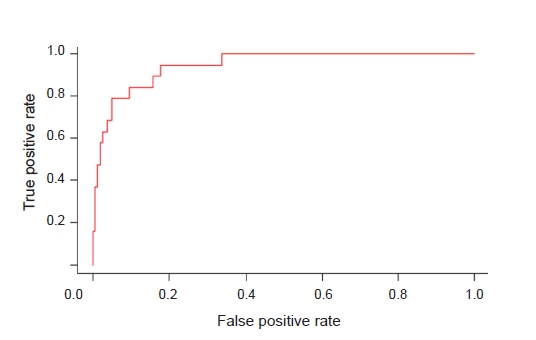
20]. In this respect, timely measurements of traditional biomarkers are useful and practical for predicting AKI. The McMahon score predicts the risk of renal failure requiring renal replacement therapy (RRT) or resulting in mortality in patients with rhabdomyolysis. The variables include age; female sex; origin of rhabdomyolysis; and initial values of creatinine, calcium, CK, and phosphate. A McMahon score of 6 points or higher has greater sensitivity and specificity than a CK level of greater than 5,000 U/L in predicting the risk of RRT [
34]. Although the McMahon score was developed for adult patients, modification of variables based on our and other pediatric studies may give us a scoring system applicable to children in future studies.
Dipstick urine OB can yield false-positive results caused by dehydration, exercise, hemoglobinuria, and myoglobinuria, which are commonly seen in rhabdomyolysis [
1,
35]. However, it is an inexpensive and rapid test that is useful for detecting the risk-estimation markers of AKI. The correlation between AKI and urine OB shown in our study supports the usefulness of the urine dipstick test. In our study, dark-colored urine did not show any difference between the AKI-status subgroups, while true hematuria was significantly higher in the AKI subgroup. Thus, the influence of hematuria on the degree of urine OB is greater than that of myoglobin in this group, and these results suggest a role of hematuria in AKI. Hematuria has a pathophysiological mechanism that is involved in aspects of renal damage, such as direct tubular damage, oxidative stress, and secretion of inflammatory cytokines, which occurs during rhabdomyolysis [
36]. Hematuria is presumed to aggravate kidney function in patients with rhabdomyolysis. Therefore, the risk of AKI could increase in cases of true hematuria and patients with higher OB scores following urinalysis.
Fluid resuscitation has been widely used to treat rhabdomyolysis, and it was also the most commonly used method in our study. Although fluid therapy is emphasized by consensus [
5,
37,
38], other treatment methods are still controversial. Dawley [
37] reported that management consists of rapidly aggressive intravenous resuscitation to maintain urine output and limited use of bicarbonate for acidosis and mannitol for oliguria, respectively. Michelsen et al. [
38] reported guidelines that recommend early fluid resuscitation using crystalloids but not the routine use of diuretics, mannitol, alkalization, or RRT. CRRT effectively removes myoglobin and manages AKI [
38]. A Cochrane systematic review concluded that CRRT provides some benefits, but the evidence is insufficient [
39]. However, significantly decreased myoglobin levels; improved BUN, creatinine, and potassium levels; and reduced oliguria and hospitalizations were reported relative to when conventional therapy was used. Mortality rates exhibited significant differences, but data on long-term outcomes are lacking [
39].
To our knowledge, this is the first large-scale pediatric multicenter study. First, our study clarified the age distribution, proportions of boys and girls, and anthropometric data in rhabdomyolysis, which have been reported in previous studies. Second, our detailing of underlying diseases and causes of rhabdomyolysis can help in understanding their influence in the pediatric population. Third, we found that the independent risk factors for AKI in rhabdomyolysis were multiorgan failure; the presence of underlying disorders; increased levels of urine OB, AST, and uric acid; and decreased levels of calcium. Unlike in previous adult studies, sex, BMI, and CK were not included as risk factors. Fourth, the role of urinalysis was newly highlighted for predicting AKI.
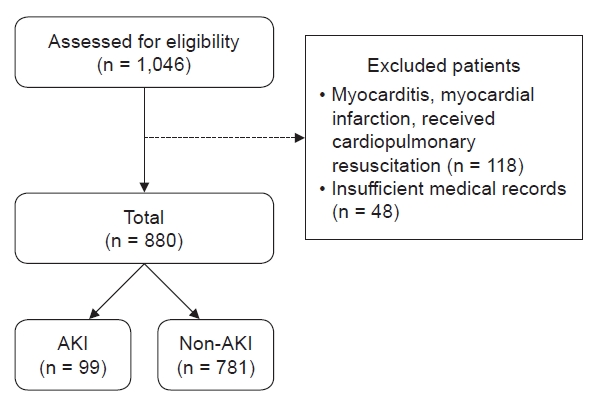
However, there are some limitations to account for as this was a retrospective multicenter study. First, some medical record data regarding etiology, underlying disorders, and laboratory data were missing, which led to the exclusion of some cases. Second, AKI and oliguria could be underestimated or overestimated because of the methodological issues with baseline creatinine level and urine output. Third, some laboratory data, such as CK and myoglobin levels and urine red blood cell count exceeding the upper reference limit, were reported only as values over the upper limit instead of the exact value, which might have led to underestimation of their influence. In addition, the analysis methods and equipment used likely varied among the involved hospitals. Fourth, underlying disorders were classified based on the involved organ or pathology of the disease for the purpose of detailed descriptions, but the criteria for classification were vague for disorders with multisystemic involvement, and severity was not considered. Thus, the interpretation of the influence of underlying disorders on rhabdomyolysis and AKI is limited.
Rhabdomyolysis is a well-known disease, but its epidemiology, risk factors, incidence rates of AKI, and mortality have not been elucidated because of the heterogeneity caused by varying definitions and etiologies. Our study revealed characteristic clinical and laboratory features of rhabdomyolysis in a multicenter Korean pediatric population as well as the predictive factors for AKI. These findings will contribute to a broader understanding of pediatric rhabdomyolysis and enable early intervention against rhabdomyolysis-induced AKI.


































 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print