| Kidney Res Clin Pract > Volume 40(4); 2021 > Article |
|
Abstract
Background
Methods
Results
Notes
Figure 1.
Flow chart for patient enrollment.

Figure 2.
Cumulative event-free survival according to use of metformin in patients with chronic kidney disease and taking diabetes medication, after propensity score matching.
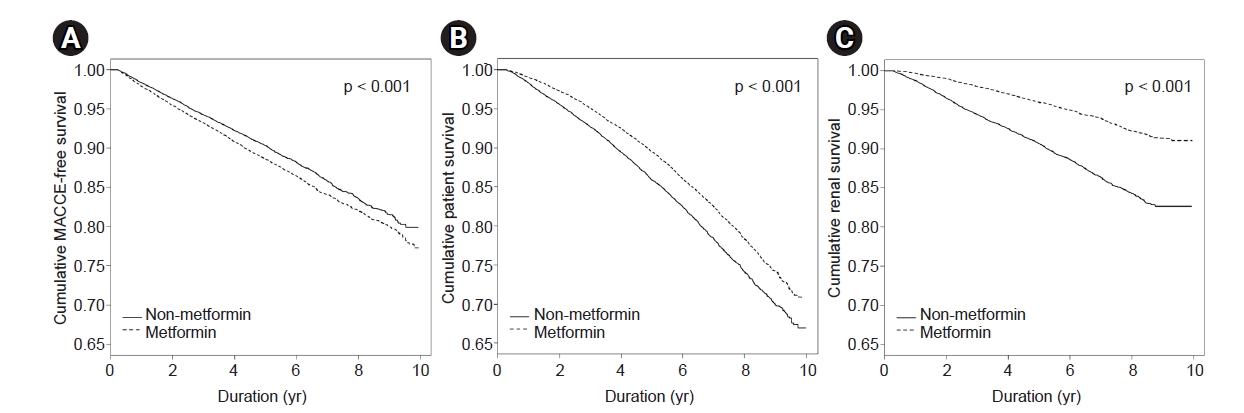
Table 1.
| Characteristic | Metformin non-users | Metformin users | p-value |
|---|---|---|---|
| No. of patients | 20,047 | 77,666 | |
| Age (yr) | 66.3 ± 9.5 | 66.0 ± 8.9 | <0.001 |
| Sex | <0.001 | ||
| Male | 13,972 (69.7) | 49,279 (63.4) | |
| Female | 6,075 (30.3) | 28,387 (36.6) | |
| Comorbid condition | |||
| Hypertension | 17,447 (87.0) | 64,528 (83.1) | <0.001 |
| Myocardial infarction | 567 (2.8) | 1,716 (2.2) | <0.001 |
| Heart failure | 1,072 (5.3) | 2,844 (3.7) | <0.001 |
| Peripheral vascular disease | 2,383 (11.9) | 9,424 (12.1) | 0.34 |
| Stroke | 3,127 (15.6) | 10,767 (13.9) | <0.001 |
| Cancer | 1,153 (5.8) | 3,419 (4.4) | <0.001 |
| Medication use | |||
| Aspirin | 7,787 (38.8) | 30,020 (38.7) | 0.62 |
| Statin | 9,218 (46.0) | 33,026 (42.5) | <0.001 |
| RASB | 14,852 (74.1) | 53,834 (69.3) | <0.001 |
| Charlson comorbidity index | 2.6 ± 1.6 | 2.2 ± 1.3 | <0.001 |
| Baseline measurement | |||
| Body mass index (g/m2) | 25.2 ± 3.2 | 25.1 ± 3.2 | <0.001 |
| Systolic BP (mmHg) | 130.9 ± 16.6 | 129.5 ± 16.0 | <0.001 |
| Diastolic BP (mmHg) | 77.1 ± 10.3 | 77.2 ± 10.0 | 0.51 |
| Fasting glucose (mg/dL) | 131.8 ± 47.0 | 136.6 ± 48.6 | <0.001 |
| Creatinine (mg/dL) | 1.7 ± 2.1 | 1.5 ± 2.3 | <0.001 |
| eGFR (mL/min/1.73 m2) | 46.1 ± 12.3 | 50.7 ± 9.6 | <0.001 |
| Total cholesterol (mg/dL) | 178.4 ± 44.1 | 180.4 ± 43.1 | <0.001 |
| HDL cholesterol (mg/dL) | 48.1 ± 18.6 | 49.2 ± 19.8 | <0.001 |
| LDL cholesterol (mg/dL) | 98.9 ± 45.1 | 99.2 ± 73.9 | 0.46 |
| Triglycerides (mg/dL) | 163.8 ± 114.9 | 168.5 ± 118.8 | <0.001 |
| Proteinuriaa | 4,665 (23.3) | 10,773 (13.9) | <0.001 |
| Smoking status | <0.001 | ||
| Non-smoker | 10,283 (51.3) | 42,231 (54.4) | |
| Ex-smoker | 5,872 (29.3) | 20,408 (26.3) | |
| Current smoker | 3,892 (19.4) | 15,027 (19.3) | |
| Alcohol consumption (unit/wk) | <0.001 | ||
| 0 | 8,565 (42.7) | 33,695 (43.4) | |
| 1–7 | 5,733 (28.6) | 21,080 (27.1) | |
| 8–14 | 2,392 (11.9) | 9,172 (11.8) | |
| ≥15 | 3,357 (16.8) | 13,719 (17.7) |
Table 2.
| Outcome | Metformin group | Event, n (%) | Incidence (per 1,000 patient-yr) |
Crude |
Adjusteda |
||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||||
| MACCE | Non-users | 2,082 (10.4) | 21.1 | (Reference) | - | (Reference) | - |
| Users | 9,352 (12.0) | 22.5 | 1.06 (1.01–1.11) | 0.021 | 1.20 (1.14–1.26) | <0.001 | |
| CHD | Non-users | 1,261 (3.3) | 12.8 | (Reference) | - | (Reference) | - |
| Users | 4,983 (6.4) | 12.0 | 0.93 (0.88–0.99) | 0.028 | 1.07 (1.00–1.14) | 0.05 | |
| Stroke | Non-users | 821 (4.1) | 8.3 | (Reference) | - | (Reference) | - |
| Users | 4,369 (5.6) | 10.5 | 1.25 (1.16–1.35) | <0.001 | 1.40 (1.30–1.51) | <0.001 | |
CHD, coronary heart disease; CI, confidence interval; HR, hazard ratio; MACCE, major adverse cardiac and cerebrovascular events.
a Adjusted for age, sex, smoking, alcohol consumption, history of hypertension, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, cancer, body mass index, systolic blood pressure, fasting glucose, estimated glomerular filtration rate, total cholesterol, presence of proteinuria, and use of aspirin, a statin, or renin-angiotensin system blockade.
Table 3.
| Outcome | Metformin group | Event, n (%) | Incidence (per 1,000 patient-yr) |
Crude |
Adjusteda |
||
|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | ||||
| All-cause mortality | Non-users | 3,370 (16.8) | 32.5 | (Reference) | - | (Reference) | - |
| Users | 9,830 (12.7) | 22.2 | 0.66 (0.63–0.68) | <0.001 | 0.78 (0.74–0.81) | <0.001 | |
| ESRD | Non-users | 1,966 (9.8) | 20.1 | (Reference) | - | (Reference) | - |
| Users | 2,116 (2.7) | 4.8 | 0.23 (0.22–0.25) | <0.001 | 0.44 (0.42–0.47) | <0.001 | |
a Adjusted for age, sex, smoking, alcohol consumption, history of hypertension, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, cancer, body mass index, systolic blood pressure, fasting glucose, estimated glomerular filtration rate, total cholesterol, presence of proteinuria, and use of aspirin, a statin, or renin-angiotensin system blockade.
Table 4.
| Outcome |
Overall (n = 40,094) |
CKD stage (eGFR, mL/min/1.73 m2) |
||||||
|---|---|---|---|---|---|---|---|---|
|
3a (≥45, <60) (n = 25,650) |
3b (≥30,<45) (n = 9,664) |
4 & 5 (<30) (n = 4,780) |
||||||
| Crude | Adjusteda | Crude | Adjusteda | Crude | Adjusteda | Crude | Adjusteda | |
| MACCE | 1.15 (1.09–1.22) | 1.15 (1.09–1.22) | 1.36 (1.25–1.47) | 1.36 (1.26–1.47) | 1.10 (0.99–1.22) | 1.10 (0.99–1.22) | 0.84 (0.73–0.97) | 0.98 (0.84–1.14) |
| CHD | 1.01 (0.93–1.09) | 1.02 (0.94–1.10) | 1.19 (1.07–1.32) | 1.19 (1.07–1.32) | 1.00 (0.87–1.15) | 1.01 (0.88–1.16) | 0.76 (0.63–0.91) | 0.95 (0.78–1.16) |
| Stroke | 1.37 (1.26–1.50) | 1.36 (1.25–1.49) | 1.60 (1.42–1.80) | 1.61 (1.43–1.81) | 1.24 (1.05–1.45) | 1.23 (1.05–1.44) | 0.99 (0.78–1.24) | 1.02 (0.80–1.31) |
| All-cause mortality | 0.78 (0.74–0.82) | 0.76 (0.73–0.80) | 0.83 (0.77–0.89) | 0.83 (0.78–0.89) | 0.87 (0.80–0.95) | 0.85 (0.78–0.93) | 0.68 (0.61–0.77) | 0.76 (0.67–0.85) |
| ESRD | 0.47 (0.44–0.51) | 0.45 (0.42–0.48) | 0.61 (0.51–0.72) | 0.64 (0.54–0.76) | 0.67 (0.59–0.76) | 0.73 (0.64–0.82) | 0.27 (0.24–0.30) | 0.41 (0.36–0.46) |
Data are expressed as hazard ratio (95% confidence interval).
CHD, coronary heart disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MACCE, major adverse cardiac and cerebrovascular events.
a Adjusted for age, sex, smoking, alcohol consumption, history of hypertension, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, cancer, body mass index, systolic blood pressure, fasting glucose, eGFR, total cholesterol, presence of proteinuria, and use of aspirin, a statin, or renin-angiotensin system blockade.
Table 5.
| Outcome |
Overall (n = 55,201) |
CKD stage (eGFR, mL/min/1.73 m2) |
||||||
|---|---|---|---|---|---|---|---|---|
|
3a (≥45, <60) (n = 43,931) |
3b (≥30,<45) (n = 8,593) |
4 & 5 (<30) (n = 2,677) |
||||||
| Crude | Adjustedb | Crude | Adjustedb | Crude | Adjustedb | Crude | Adjustedb | |
| MACCE | 1.39 (1.32–1.47) | 1.66 (1.57–1.76) | 1.68 (1.56–1.81) | 1.81 (1.69–1.95) | 1.50 (1.35–1.67) | 1.67 (1.50–1.87) | 0.95 (0.79–1.15) | 1.21 (0.96–1.52) |
| CHD | 1.23 (1.15–1.32) | 1.47 (1.36–1.58) | 1.49 (1.36–1.64) | 1.58 (1.44–1.74) | 1.27 (1.10–1.46) | 1.49 (1.28–1.73) | 0.90 (0.71–1.15) | 1.23 (0.92–1.66) |
| Stroke | 1.65 (1.51–1.79) | 1.96 (1.80–2.15) | 1.97 (1.76–2.21) | 2.18 (1.94–2.45) | 1.85 (1.57–2.17) | 1.93 (1.63–2.29) | 1.03 (0.76–1.40) | 1.19 (0.82–1.73) |
| All-cause mortality | 0.70 (0.67–0.74) | 0.88 (0.83–0.93) | 0.80 (0.75–0.86) | 0.90 (0.84–0.96) | 0.98 (0.89–1.08) | 1.08 (0.97–1.20) | 0.65 (0.54–0.77) | 0.96 (0.78–1.19) |
| ESRD | 0.12 (0.11–0.13) | 0.29 (0.26–0.33) | 0.30 (0.25–0.36) | 0.47 (0.38–0.57) | 0.26 (0.21–0.31) | 0.60 (0.49–0.74) | 0.13 (0.11–0.17) | 0.23 (0.18–0.29) |
Data are expressed as hazard ratio (95% confidence interval).
CHD, coronary heart disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MACCE, major adverse cardiac and cerebrovascular events.
b Adjusted for age, sex, smoking, alcohol consumption, history of hypertension, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, cancer, body mass index, systolic blood pressure, fasting glucose, eGFR, total cholesterol, presence of proteinuria, and use of aspirin, a statin, or renin-angiotensin system blockade.
Table 6.
| Outcome |
Overall (n = 32,744) |
CKD stage (eGFR, mL/min/1.73 m2) |
||||||
|---|---|---|---|---|---|---|---|---|
|
3a (≥45, <60) (n = 21,698)) |
3b (≥30,<45) (n = 7,690)) |
4 & 5 (<30) (n = 1,998)) |
||||||
| Crude | Adjustedb | Crude | Adjustedb | Crude | Adjustedb | Crude | Adjustedb | |
| MACCE | 1.59 (1.49–1.69) | 1.65 (1.55–1.75) | 1.78 (1.64–1.93) | 1.81 (1.67–1.97) | 1.53 (1.37–1.70) | 1.67 (1.49–1.87) | 1.01 (0.81–1.25) | 1.34 (1.03–1.75) |
| CHD | 1.39 (1.29–1.51) | 1.46 (1.35–1.59) | 1.54 (1.38–1.71) | 1.57 (1.40–1.75) | 1.34 (1.16–1.55) | 1.50 (1.29–1.75) | 0.92 (0.70–1.21) | 1.30 (0.93–1.82) |
| Stroke | 1.89 (1.72–2.08) | 1.92 (1.75–2.12) | 2.17 (1.90–2.47) | 2.20 (1.94–2.51) | 1.80 (1.52–2.13) | 1.91 (1.61–2.28) | 1.19 (0.83–1.69) | 1.43 (0.93–2.22) |
| All-cause mortality | 0.88 (0.82–0.93) | 0.89 (0.84–0.95) | 0.87 (0.80–0.95) | 0.87 (0.80–0.94) | 1.02 (0.92–1.13) | 1.09 (0.98–1.22) | 0.66 (0.54–0.81) | 1.00 (0.79–1.27) |
| ESRD | 0.22 (0.19–0.24) | 0.30 (0.26–0.33) | 0.41 (0.32–0.52) | 0.45 (0.35–0.57) | 0.28 (0.23–0.35) | 0.60 (0.48–0.73) | 0.13 (0.11–0.17) | 0.26 (0.20–0.34) |
Data are expressed as hazard ratio (95% confidence interval).
CHD, coronary heart disease; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; MACCE, major adverse cardiac and cerebrovascular events.
b Adjusted for age, sex, smoking, alcohol consumption, history of hypertension, myocardial infarction, congestive heart failure, peripheral vascular disease, stroke, cancer, body mass index, systolic blood pressure, fasting glucose, eGFR, total cholesterol, presence of proteinuria, and use of aspirin, a statin, or renin-angiotensin system blockade.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Min-Ho Kim

https://orcid.org/0000-0003-4909-2308Hyung Jung Oh

https://orcid.org/0000-0002-4281-696XSoon Hyo Kwon

https://orcid.org/0000-0002-4114-4196Jin Seok Jeon

https://orcid.org/0000-0003-2421-2289Hyunjin Noh

https://orcid.org/0000-0002-1904-1684Dong Cheol Han

https://orcid.org/0000-0002-8835-8642Hyoungnae Kim

https://orcid.org/0000-0002-5359-0214Dong-Ryeol Ryu

https://orcid.org/0000-0002-5309-7606 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















