| Kidney Res Clin Pract > Volume 41(4); 2022 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This research was supported by funding (2014-ER6301-00, 2014-ER6301-01, 2014-ER6301-02, 2017-ER6301-00, 2017-ER6301-01, and 2017-ER6301-02) provided by the Research of Korea Centers for Disease Control and Prevention Agency. This study was supported by a new faculty research seed money grant of Yonsei University College of Medicine for 2021 (2021-32-0079).
Authors’ contributions
Conceptualization: MSK, JCJ, CA, JY
Data curation: HJJ, MKJ, DWC, SJNC, MSK, JHR, JCJ, JY
Formal analysis: HJJ, TYK, JHR, JCJ, JY
Investigation: TYK, MKJ, DWC, SJNC, MSK, JHR, CA, JY
Methodology: MSK, JCJ, CA, JY
Writing–original draft: HJJ, JY
Writing–review & editing: All authors
All authors read and approved the final manuscript.
Supplementary Materials
Acknowledgments
Figure 1.
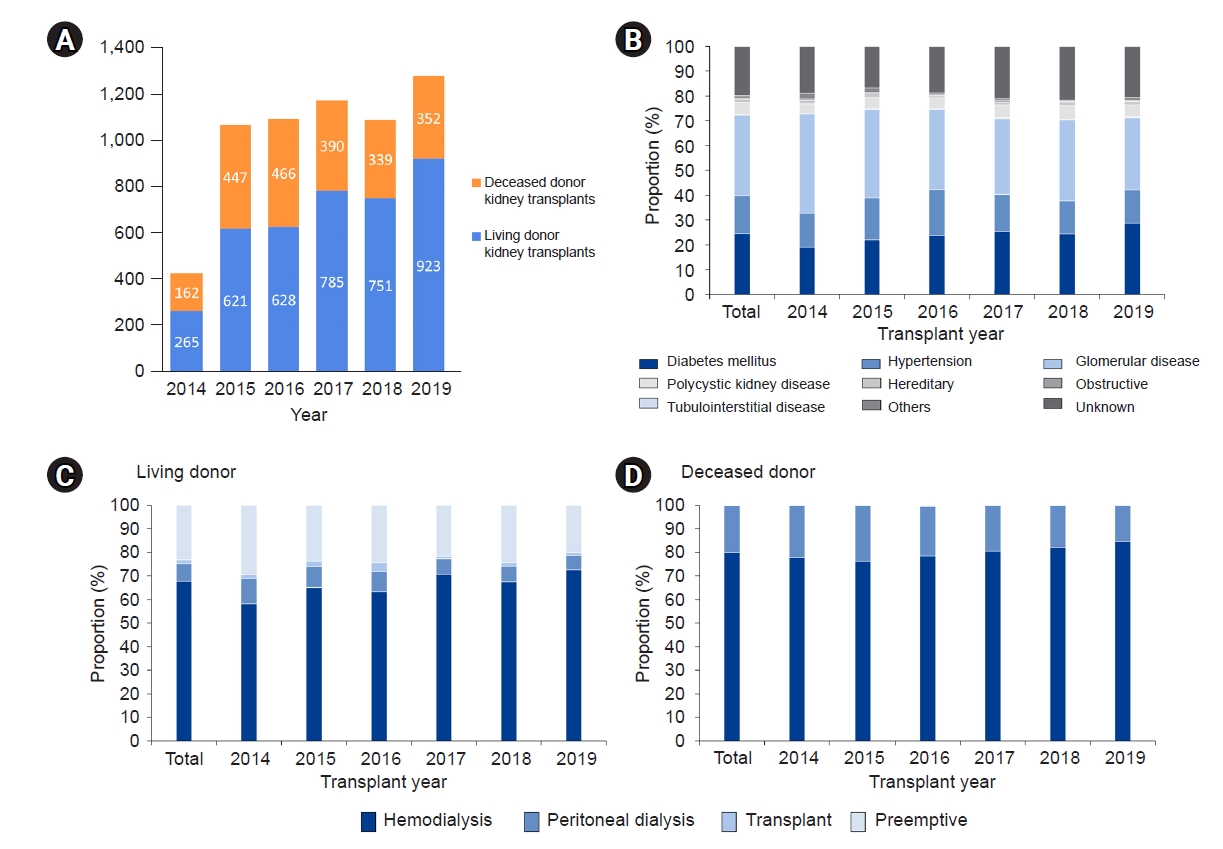
Figure 2.

Figure 3.
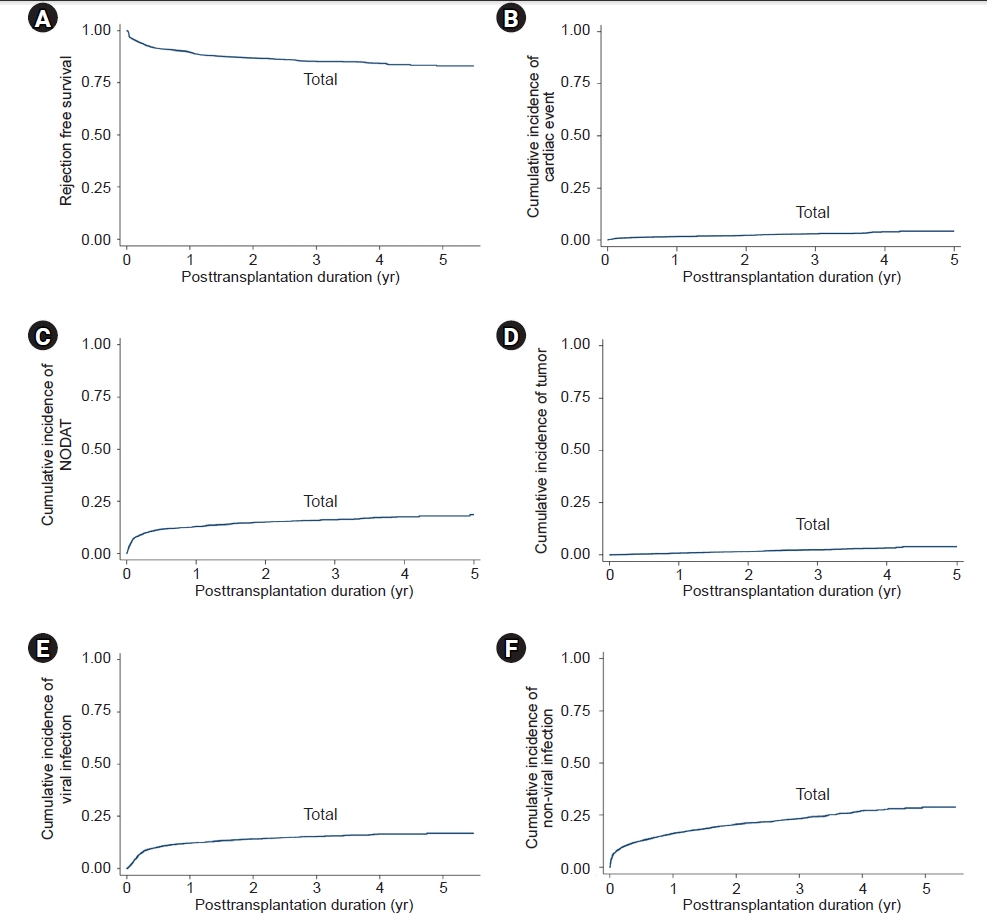
Figure 4.
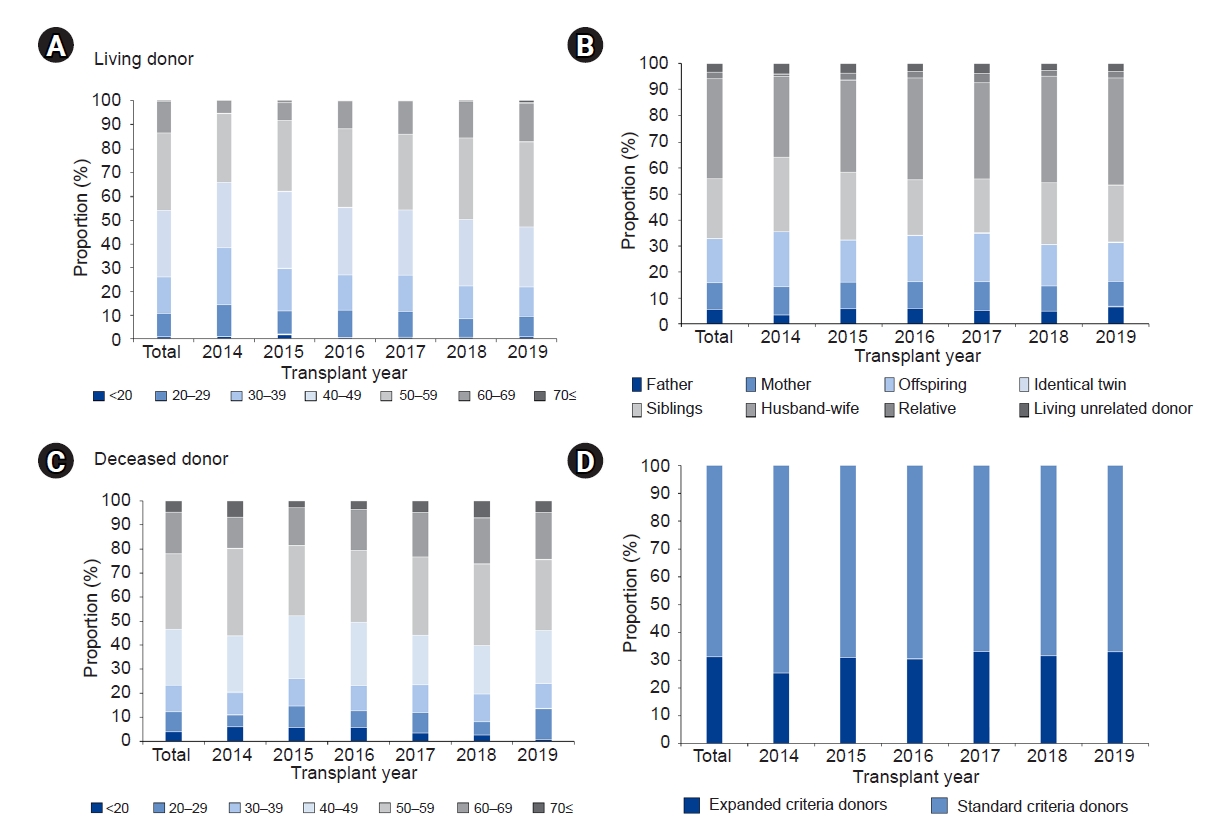
Table 1.
Values are presented as number only, mean ± standard deviation, number (%), or median (interquartile range). The chi-square test was performed to evaluate differences in categorical variables, and the Student t-test or analysis of variance test was conducted to evaluate differences in continuous variables.
ATG, antithymocyte globulin; DDKT, deceased-donor kidney transplantation; eGFR, estimated glomerular filtration rate; ESRD, end-stage renal disease; HBsAg, hepatitis B surface antigen; HCV Ab, hepatitis C virus antibody; HLA, human leukocyte antigen; KT, kidney transplantation; LDKT, living-donor kidney transplantation.
Table 2.
| Variable | Adjusted HR (95% CI) | p-value |
|---|---|---|
| Recipient age | 1.047 (1.017–1.078) | 0.002* |
| History of cardiovascular disease, vs. no | 2.525 (1.460–4.364) | 0.001* |
| Bortezomib use, vs. no | 11.559 (1.523–87.708) | 0.02* |
| Tacrolimus, vs. cyclosporin | 0.234 (0.089–0.617) | 0.006* |
| Use of antimetabolite drug, vs. no | 0.180 (0.090–0.360) | <0.001* |
| Serum creatinine at discharge | 1.476 (1.247–1.747) | <0.001* |
| Living donor, vs. deceased donor | 0.218 (0.098–0.483) | <0.001* |
CI, confidence interval; HR, hazard ratio.
Adjusted for recipient age and sex, donor age and sex, number of previous transplants, dialysis modality, desensitization, recipient body mass index, recipient smoking history, recipient history of diabetes, recipient history of hypertension, history of cardiovascular disease, history of tumor, usage of statins, number of human leukocyte antigen mismatches, antithymocyte globulin induction, bortezomib use, tacrolimus (vs. cyclosporine) at discharge, antimetabolite drug at discharge, mechanistic target of rapamycin inhibitor at discharge, recipient serum creatinine concentration at discharge, donor type (living vs. deceased), donor history of diabetes, donor history of hypertension, donor serum creatinine concentration at baseline, acute T-cell–mediated rejection, and acute antibody-mediated rejection. Antimetabolite drugs include mycophenolate, mizoribine, and azathioprine.
Table 3.
| Factor | Adjusted HR (95% CI) | p-value |
|---|---|---|
| Use of antimetabolite drug, vs. no | 0.333 (0.161–0.687) | 0.003* |
| Serum creatinine at discharge | 1.906 (1.683–2.158) | <0.001* |
| Acute T-cell–mediated rejection, vs. no | 4.783 (2.870–7.970) | <0.001* |
| Acute antibody-mediated rejection, vs. no | 5.103 (2.945–8.841) | <0.001* |
CI, confidence interval; HR, hazard ratio.
Adjusted for recipient age and sex, donor age and sex, number of previous transplants, dialysis modality, desensitization, recipient body mass index, recipient smoking, recipient history of diabetes, recipient history of hypertension, history of cardiovascular disease, history of tumor, usage of statins, number of human leukocyte antigen mismatches, antithymocyte globulin induction, bortezomib use, tacrolimus (vs. cyclosporine) at discharge, antimetabolite drug at discharge, mechanistic target of rapamycin inhibitor at discharge, recipient serum creatinine concentration at discharge, donor type (living vs. deceased), donor history of diabetes, donor history of hypertension, donor serum creatinine concentration at baseline, acute T-cell–mediated rejection, and acute antibody-mediated rejection. Antimetabolite drugs include mycophenolate, mizoribine, and azathioprine.
Table 4.
| Factor | Adjusted HR (95% CI) | p-value |
|---|---|---|
| Recipient age | 0.981 (0.972–0.991) | <0.001* |
| Donor age | 1.015 (1.006–1.023) | 0.001* |
| HLA-incompatibility, vs. no | 1.599 (1.159–2.206) | 0.004* |
| No. of HLA mismatches | 1.154 (1.083–1.230) | <0.001* |
CI, confidence interval; HLA, human leukocyte antigen; HR, hazard ratio.
Adjusted for recipient age and sex, donor age and sex, number of previous transplants, dialysis modality, desensitization, recipient body mass index, recipient smoking, recipient history of diabetes, recipient history of hypertension, history of cardiovascular disease, history of tumor, usage of statins, number of HLA mismatches, antithymocyte globulin induction, bortezomib use, tacrolimus (vs. cyclosporine) at discharge, antimetabolite drug at discharge, mechanistic target of rapamycin inhibitor at discharge, recipient serum creatinine concentration at discharge, donor type (living vs. deceased), history of donor diabetes, history of donor hypertension, and donor serum creatinine concentration at baseline.
Table 5.
| Factor | Adjusted HR (95% CI) | p-value |
|---|---|---|
| History of previous transplantation | 8.642 (1.322–56.492) | 0.02* |
| Diabetes | 4.448 (1.009–19.609) | 0.049* |
| History of cardiovascular disease | 7.384 (1.619–33.665) | 0.01* |
CI, confidence interval; HR, hazard ratio.
Adjusted for recipient age and sex, donor age and sex, number of previous transplants, dialysis modality, desensitization, recipient body mass index, recipient smoking, recipient history of diabetes, recipient history of hypertension, history of cardiovascular disease, history of tumor, usage of statins, number of human leukocyte antigen mismatches, antithymocyte globulin induction, bortezomib use, tacrolimus (vs. cyclosporine) at discharge, antimetabolite drug at discharge, mechanistic target of rapamycin inhibitor at discharge, recipient serum creatinine concentration at discharge, donor type (living vs. deceased), history of donor diabetes, history of donor hypertension, and donor serum creatinine concentration at baseline.
Table 6.
| Factor | Adjusted HR (95% CI) | p-value |
|---|---|---|
| Donor age | 1.026 (1.018–1.034) | <0.001* |
| Body mass index | 1.041 (1.013–1.069) | 0.003* |
| ATG induction, vs. basiliximab | 1.418 (1.145–1.757) | 0.001* |
| Living donor, vs. deceased donor | 0.703 (0.543–0.909) | 0.007* |
ATG, antithymocyte globulin; CI, confidence interval; HR, hazard ratio.
Adjusted for recipient age and sex, donor age and sex, number of previous transplants, dialysis modality, desensitization, recipient body mass index, recipient smoking, recipient history of diabetes, recipient history of hypertension, history of cardiovascular disease, history of tumor, usage of statins, number of human leukocyte antigen mismatches, ATG induction, bortezomib use, tacrolimus (vs. cyclosporine) at discharge, antimetabolite drug at discharge, mechanistic target of rapamycin inhibitor at discharge, recipient serum creatinine concentration at discharge, donor type (living vs. deceased), history of donor diabetes, history of donor hypertension, and donor serum creatinine concentration at baseline. Antimetabolite drugs include mycophenolate, mizoribine, and azathioprine.
Table 7.
| Variable | Adjusted HR (95% CI) | p-value |
|---|---|---|
| Female recipient, vs. male | 1.814 (1.510–2.180) | <0.001 |
| Cause of desensitization | ||
| HLA incompatible, vs. no | 1.479 (1.154–1.895) | 0.002* |
| ABO incompatible, vs. no | 1.330 (1.045–1.693) | 0.02* |
| History of cardiovascular disease, vs. no | 1.635 (1.312–2.036) | <0.001* |
| Mycophenolate + mizoribine + azathioprine, vs. no | 0.689 (0.486–0.978) | 0.04* |
CI, confidence interval; HLA, human leukocyte antigen; HR, hazard ratio.
Adjusted for recipient age and sex, donor age and sex, number of previous transplants, dialysis modality, cause of desensitization (HLA incompatible/ABO incompatible vs. no), recipient body mass index, recipient smoking, recipient history of diabetes, recipient history of hypertension, history of cardiovascular disease, history of tumor, usage of statins, number of HLA mismatches, antithymocyte globulin induction, bortezomib use, tacrolimus (vs. cyclosporine) at discharge, antimetabolite drug at discharge, mechanistic target of rapamycin inhibitor at discharge, recipient serum creatinine concentration at discharge, donor type (living vs. deceased), history of donor diabetes, history of donor hypertension, and donor serum creatinine concentration at baseline. Antimetabolite drugs include mycophenolate, mizoribine, and azathioprine.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Hee Jung Jeon

https://orcid.org/0000-0003-3264-8525Tai Yeon Koo

https://orcid.org/0000-0003-2014-7723Man Ki Ju

https://orcid.org/0000-0002-4112-7003Dong-Wan Chae

https://orcid.org/0000-0001-9401-892XSoo Jin Na Choi

https://orcid.org/0000-0002-0179-731XMyoung Soo Kim

https://orcid.org/0000-0002-8975-8381Jung-Hwa Ryu

https://orcid.org/0000-0002-1648-624XJong Cheol Jeon

https://orcid.org/0000-0003-0301-7644Curie Ahn

https://orcid.org/0000-0001-7033-1102Jaeseok Yang

https://orcid.org/0000-0002-5378-7797 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print