| Kidney Res Clin Pract > Volume 33(3); 2014 > Article |
|
Abstract
Background
Urinary kidney injury molecule-1 (KIM-1) is an early and sensitive biomarker of acute kidney injury, but it is unclear if it is a biomarker of chronic glomerulonephritis. We evaluated whether urinary KIM-1 levels in patients with immunoglobulin A (IgA) nephropathy can be a marker to reflect clinicopathological severity and predict the prognosis.
Methods
We measured urinary KIM-1 levels in 40 patients (15 males; mean age 36.6±12.9 years) with IgA nephropathy and 10 healthy people (5 males; mean age 37.3±9.6 years) as controls. The correlation of urinary KIM-1 levels with patients’ clinical parameters, histological grades, and follow-up data were analyzed using the modified H. S. Lee grading system and tubulointerstitial change scores.
Results
Urinary KIM-1 levels were higher in patients with IgA nephropathy than healthy controls (P=0.001). Univariate and multivariate regression analyses showed that urinary KIM-1 levels had a direct correlation with H. S. Lee grade and tubulointerstitial inflammation (P=0.004 and P=0.011, respectively).
Conclusion
In patients with IgA nephropathy, urinary KIM-1 has a significant correlation with histopathologic severity.
Keyword
IgA nephropathy, Kidney biomarkers, Kidney injury molecule-1 (KIM-1)Immunoglobulin A (IgA) nephropathy was first described by Berger and Hinglais [1] in 1968 and is now the most common glomerulonephritis in the world, comprising about 20–45% of primary glomerulonephritis [2]. The clinical course and prognosis of IgA nephropathy is highly variable, ranging from asymptomatic urinary abnormalities to severe, rapid decline in kidney function. About 30% of patients are reported to progress to end-stage renal disease (ESRD) in 20–25 years [3].
Arterial hypertension, persistent proteinuria, elevated baseline serum creatinine level, male sex, and older age at disease onset are known markers of poor prognosis in IgA nephropathy [4], [5], [6], [7], [8]. Several studies have reported that the severity and prognosis of IgA nephropathy are correlated with the expression levels of specific markers such as serum erythropoietin, urinary N-acetyl-β-D-glucosaminidase (NAG), beta-2-microglobulin, and nestin [9], [10], [11]; however, none of these markers are sensitive or specific. Predicting which patients with IgA nephropathy will progress to ESRD remains controversial.
Histopathologic features such as glomerular sclerosis, tubular atrophy, extension of immune deposits to the perivascular space, and crescent formation may also aid in predicting risk for progression of IgA nephropathy [12]. Recently, tubulointerstitial injury rather than glomerular injury is thought to be the final pathway to ESRD; therefore, biomarkers reflecting tubulointerstitial injury are currently being studied.
Kidney injury molecule-1 (KIM-1) is a type I transmembrane glycoprotein with an ectodomain containing an immunoglobulin-like domain and a mucin domain. This protein is usually undetectable in normal kidneys but is expressed at high levels in proximal tubular cells after ischemic or nephrotoxic injury as well as other forms of injury including contrast-induced nephropathy and renal allograft rejection [13], [14]. A recent study suggests that KIM-1 contributes not only to the regeneration process of tubular epithelial cells through cell dedifferentiation in acute kidney damage but also to tubular fibrosis in some chronic renal disease. Another study demonstrated that KIM-1 is upregulated in the tubules and is associated with renal fibrosis and inflammation in patients with various chronic proteinuric kidney disease [15]. Few studies, however, have linked KIM-1 with IgA nephropathy.
We therefore evaluated urinary KIM-1 in patients with IgA nephropathy to determine its ability to reflect clinicopathological severity and predict the response to treatment better than baseline clinical and histopathologic parameters.
This study is part of an IgA nephropathy cohort study about the effect of treatment and role of biomarkers in IgA nephropathy in our hospital. We enrolled 40 patients with biopsy-proven IgA nephropathy who visited Kyung Hee University Hospital, Seoul, Korea between 2007 and June 2009, excluding those patients with acute kidney injury, renal replacement therapy, or urinary tract infections. We compared these patients with healthy controls with an estimated glomerular filtration rate (eGFR) of more than 90 mL/min/1.73 m2 and no apparent renal damage.
We recorded patients’ age and sex, measured their height, weight, and blood pressure, and assessed blood urea nitrogen (BUN), serum creatinine (sCr), hemoglobin, albumin, total cholesterol, triglyceride, urinary protein, and creatinine levels. eGFR was calculated using the modification of diet in renal disease study (MDRD) equation. All the samples were collected prior to the commencement of immunosuppressive drugs. Urine samples were collected from the first spot urine on the morning of the day of renal biopsy and stored at −70oC. Proteinuria is expressed as urinary protein/creatinine ratio (g/g Cr, UPCR).
Urine red blood cells were classified as Grades I–V: Grade I, 0–1/high-power field (HPF); Grade II, 2–4/HPF; Grade III, 5–9/HPF; Grade IV, 10–29/HPF; and Grade V, many/HPF.
Urinary KIM-1 levels were measured using a human quantitative commercial enzyme-linked immunosorbent assay kit (Cusabio Biotech Co., Hubei, China). Urine creatinine was measured in same urine specimens, and adjusted urinary KIM-1 levels were expressed as concentration of KIM-1/concentration of creatinine (ng/mg Cr).
IgA nephropathy pathologic findings were classified using the modified H. S. Lee grading system [16]. The percentage of glomeruli affected by crescents/segmental sclerosis/global sclerosis was specifically defined for Grades I–V. The grading was as follows: Grade I, normal or focal mesangial cell proliferation; Grade II, diffuse mesangial cell proliferation or <25% of glomeruli with crescent (Cr)/segmental sclerosis (SS)/global sclerosis (GS); Grade III, 25–49% of glomeruli with Cr/SS/GS; Grade IV, 50–75% of glomeruli with Cr/SS/GS; and Grade V, >75% of glomeruli with Cr/SS/GS. In addition to the glomerular findings, the tubulointerstitial grading score was based on the degree of fibrosis and inflammation in Grades 0–IV as follows: Grade 0, no fibrosis or inflammation; Grade I, focal fibrosis or inflammation; Grade II, mild global fibrosis or inflammation; Grade III, moderate global fibrosis or inflammation; and Grade IV, severe global fibrosis or inflammation.
sCr and UPCR were measured at 6 months after treatment. We also measured final sCr and UPCR of 31 patients who had achieved at least 2 years of a follow-up visit for the evaluation of long-term prognosis.
Data are expressed as mean±standard deviation. Correlations between urinary KIM-1 levels and biochemical and clinical parameters were investigated by performing linear and multiple regression analyses. We used a two-sample t test and a χ2 test to compare continuous variables and categorical variables, respectively. A Kruskal-Wallis test was used to compare urinary KIM-1 levels among histologic stages. To analyze disease progression, multiple regression analysis was performed using changes in eGFR and proteinuria after treatment as independent variables. A P value <0.05 was considered significant. SPSS version 15.0 for Windows (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
We investigated 40 patients with IgA nephropathy and 10 healthy controls. The mean initial sCr level of patients with IgA nephropathy was not significantly different from the mean level in the control group, but eGFR was significantly lower in the IgA nephropathy patient group than the control group (81.6±29.7 mL/min/1.73 m2 versus 112.4±11.5 mL/min/1.73 m2, P=0.01). The mean histologic stage for the patients with IgA nephropathy was 2.03±0.87 (H. S. Lee grading; Fig. 1). The mean follow-up duration was 49.55 months. The mean urinary KIM-1 levels were significantly higher in IgA nephropathy patients than controls (1.17±1.51 ng/mg Cr vs. 0.29±0.20 ng/mg Cr, P=0.001; Table 1, Fig. 2).
In univariate analysis, urinary KIM-1 levels were inversely proportional to body mass index (BMI; r=−0.382, P=0.018) but had no association with initial serum Cr, albumin, eGFR, UPCR, or uRBC grade. In multivariate analysis, BMI had no statistical association with urinary KIM-1. As a predictor of response to treatment, urinary KIM-1 levels showed no association with changes in eGFR or UPCR after treatment (Table 2).
When we investigated possible associations between urinary KIM-1 levels and histopathologic grades, urinary KIM-1 levels were directly proportional to H. S. Lee Grades I–IV (Grade I, 0.61±0.48 ng/mg Cr; Grade II, 0.85±0.97 ng/mg Cr; Grade III, 1.50±2.34 ng/mg Cr; and Grade IV, 3.81±1.24 ng/mg Cr, P=0.023) and to tubulointerstitial inflammation from Grade 0 to Grade III (Grade 0, 0.74±0.59 ng/mg Cr; Grade I, 1.04±1.59 ng/mg Cr; Grade II, 1.41±0.62 ng/mg Cr; Grade III, 3.81±1.24 ng/mg Cr, P=0.049; Fig. 3).
Our findings indicate that mean urinary KIM-1 levels, a measure of tubular function, were significantly higher in patients with IgA nephropathy than in healthy controls. eGFR was also lower in patients with IgA nephropathy compared with the control group, but because there was no correlation between eGFR and urinary KIM-1 levels in univariate analysis, we concluded urinary KIM-1 levels are elevated in patients with IgA nephropathy regardless of eGFR. Though IgA nephropathy is a glomerular process, a tubular marker may be useful because glomerular lesions induce tubular lesions through filtered toxic substances or hypertensive hemodynamic changes [17]. Inflammatory mechanisms are involved in the pathogenesis of tubular lesions by increasing levels of urinary cytokines or growth factors [18]. This pathophysiology can be associated with the increase in the secretion of these proteins observed in this study.
We also found that urinary KIM-1 levels had a significant correlation with pathologic findings such as the H. S. Lee grading score and tubulointerstitial inflammation score in patients with IgA nephropathy. Urinary KIM-1 levels as well as the H. S. Lee glomerular grading scores were higher in patients with more severe tubulointerstitial inflammation. Our data show that even after adjustment with H. S. Lee grade, urinary KIM-1 levels and tubulointerstitial inflammation were directly related, suggesting urinary KIM-1 levels are a reflection of tubulointerstitial injury. These data in IgA nephropathy are consistent with previous results that demonstrated upregulation of KIM-1 in patients with various renal diseases and an association with renal fibrosis and inflammation [15]. This study suggests that urinary KIM-1 may serve as a biomarker to assess the severity of IgA nephropathy.
Our study revealed that urinary KIM-1 levels correlated with H. S. Lee grade and tubulointerstitial inflammation but not with any other clinical variables. It is possible, therefore, that urinary KIM-1 levels can be used as a prognostic marker even in patients with IgA nephropathy and clinically normal parameters. To confirm this finding, more long-term follow-up is needed.
A recent study demonstrated the relationship between KIM-1 and the severity of IgA nephropathy. In this study, a total of 202 patients with IgA nephropathy were included, and there was a positive correlation between urinary KIM-1 levels and the severity of tubulointerstitial fibrosis and infiltration [19]. These findings are similar to our result, but we included patients with mild renal dysfunction and proteinuria, and found correlation between urinary KIM-1 levels and histopathologic severity. So our data can suggest that even if patients with IgA nephropathy have a relatively mild renal dysfunction and proteinuria, urinary KIM-1 levels might be thought to estimate the degree of glomerular injury and tubulointerstitial inflammation.
In contrast to previous studies in patients with various chronic renal diseases, patients with IgA nephropathy in our study demonstrated no correlation between urinary KIM-1 levels and initial sCr. Instead, urinary KIM-1 levels showed weak correlations with eGFR and proteinuria, but these were not statistically significant. One explanation is that we included patients with only mild renal dysfunction and proteinuria, unlike the previous study that included patients with proteinuria >2.0 g/d and sCr levels >2.0 mg/dL [15], [19].
In summary, our results indicate that urinary KIM-1 level can be used as a noninvasive marker of pathologic severity in patients with IgA nephropathy. Because the number of patients enrolled in this study is small, future prospective studies with a larger number of patients are required to confirm the association between these markers and the pathophysiology and progression of IgA nephropathy.
References
3. D׳Amico G. Natural history of idiopathic IgA nephropathy: role of clinical and histological prognostic factors. Am J Kidney Dis 36:2000;227–237.


4. Daniel L, Saingra Y, Giorgi R, Bouvier C, Pellissier JF, Berland Y. Tubular lesions determine prognosis of IgA nephropathy. Am J Kidney Dis 35:2000;13–20.


5. Bartosik LP, Lajoie G, Sugar L, Cattran DC. Predicting progression in IgA nephropathy. Am J Kidney Dis 38:2001;728–735.


6. Nozawa R, Suzuki J, Takahashi A, Isome M, Kawasaki Y, Suzuki S, Suzuki S. Clinicopathological features and the prognosis of IgA nephropathy in Japanese children on long-term observation. Clin Nephrol 64:2005;171–179.


7. Manno C, Strippoli GF, D׳Altri C, Torres D, Rossini M, Schena FP. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. Am J Kidney Dis 49:2007;763–775.


8. Reich HN, Troyanov S, Scholey JW, Cattran DC. Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18:2007;3177–3183.


9. Machiguchi T, Yoshida H, Yonemoto S, Minakata T, Nomura K, Muso E, Tamura T, Sasayama S. Does circulating erythropoietin reflect progression of IgA nephropathy? Comparison with urinary N-acetyl-beta-D-glucosaminidase. Nephrol Dial Transplant 14:1999;635–640.


10. Peters HP, van den Brand JA, Wetzels JF. Urinary excretion of low-molecular- weight proteins as prognostic markers in IgA nephropathy. Neth J Med 67:2009;54–61.

11. Tomioka M, Hiromura K, Sakairi T, Takeuchi S, Maeshima A, Kaneko Y, Kuroiwa T, Takeuchi T, Nojima N. Nestin is a novel marker for renal tubulointerstitial injury in immunoglobulin A nephropathy. Nephrology 15:2010;568–574.


12. Walsh M, Sar A, Lee D, Yilmaz S, Benediktsson H, Manns B, Hemmelgarn B. Histopathologic features aid in predicting risk for progression of IgA nephropathy. Clin Am Soc Nephrol 5:2010;425–430.

13. Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int 62:2002;237–244.


14. van Timmeren MM, Vaidya VS, van Ree RM, Oterdoom LH, de Vries AP, Gans RO, van Goor H, Stegeman CA, Bonventre JV, Bakker SJ. High urinary excretion of kidney injury molecule-1 is an independent predictor of graft loss in renal transplant recipients. Transplantation 84:2007;1625–1630.



15. van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA.. Tubular kidney injury molecule-1 (KIM-1) in human renal disease. J Pathol 212:2007;209–217.


16. Lee HS, Lee MS, Lee SM, Lee SY, Lee ES, Lee EY, Park SY, Han JS, Kim S, Lee JS. Histological grading of IgA nephropathy predicting renal outcome: revisiting H. S. Lee׳s glomerular grading system. Nephrol Dial Transplant 20:2005;342–348.


17. Koyama A, Igarashi M, Kobayashi M. Natural history and risk factors for immunoglobulin A nephropathy in Japan. Research Group on Progressive Renal Diseases. Am J Kidney Dis 29:1997;526–532.


Figure 1
Themean histopathologic stages for patients with IgA nephropathy. IgA, immunoglobulin A; TIF, tubulointerstitial fibrosis; TII, tubulointerstitial inflammation.
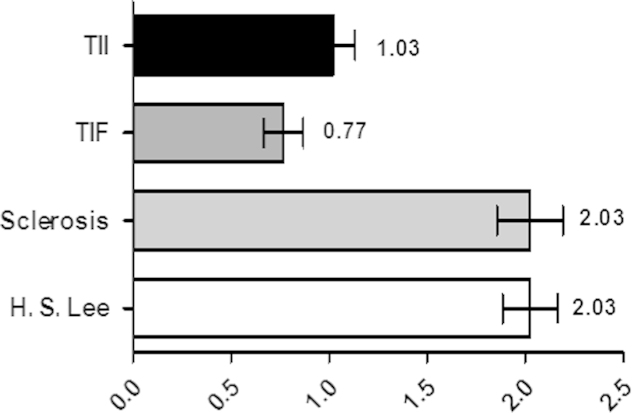
Figure 2
The mean levels of urinary KIM-1 in patients with IgA nephropathy and healthy controls. ⁎P=0.001 compared with healthy controls. Cr, creatinine; IgA, immunoglobulin A; KIM-1, kidney injury molecule-1.
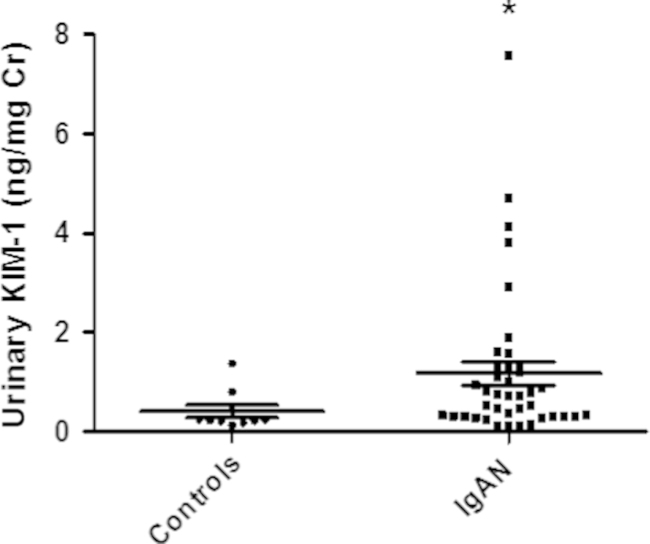
Figure 3
Thelevels of urinaryKIM-1 in patients with IgA nephropathy. (A) H. S. Lee grade and (B) tubulointerstitial inflammation. ⁎P <0.05 compared with healthy control. †P <0.05 compared with Grade I and Grade II. ‡P <0.05 compared with Grade 0 and Grade I. Cr, creatinine; IgA, immunoglobulin A; KIM, kidney injury molecule.
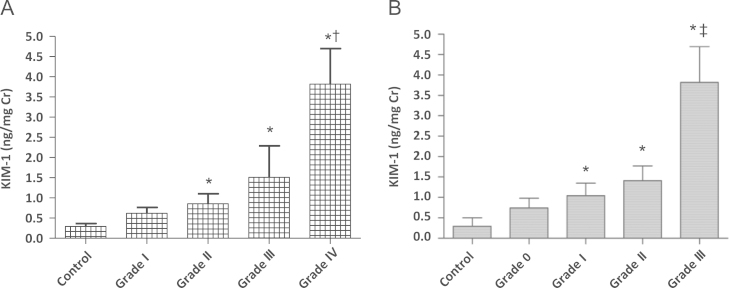
Table 1
Clinical characteristics of patients with IgA nephropathy and healthy controls
Table 2
Univariate and multivariate regression analysis results of the association between various clinical variables and urinary KIM-1 levels
|
Univariate |
Multivariate⁎ |
|||||
|---|---|---|---|---|---|---|
| Unstandardized | t | P | Unstandardized | t | P | |
| /standardized β | /standardized β | |||||
| Serum Cr, initial | 0.137/0.076 | 0.468 | 0.643 | |||
| eGFR, initial | −0.005/−0.091 | −0.563 | 0.577 | |||
| ΔeGFR 6m† | 2.772/0.150 | 1.024 | 0.313 | |||
| ΔeGFR final† | −0.004/−0.102 | −0.545 | 0.585 | |||
| UPCR, initial | 0.091/0.089 | 0.551 | 0.585 | |||
| ΔUPCR 6m† | 0.132/0.130 | 1.768 | 0.087 | |||
| ΔUPCR final† | 0.058/0.060 | 0.320 | 0.751 | |||
| H. S. Lee grade | 0.589/0.336 | 2.174 | 0.036 | 0.774/0.442 | 3.050 | 0.004 |
| Glomerulosclerosis | 0.303/0.206 | 1.280 | 0.209 | |||
| TIF | 0.391/0.160 | 0.986 | 0.331 | |||
| TII | 0.818/0.358 | 2.329 | 0.025 | 0.888/0.388 | 2.694 | 0.011 |
| Age | −0.026/−0.224 | −1.420 | 0.164 | |||
| Sex | 0.834/0.271 | 1.735 | 0.091 | |||
| BMI | −0.172/−0.382 | −2.484 | 0.018 | −0.124/−0.268 | −1.825 | 0.078 |
| Albumin | −0.448/−0.175 | −1.079 | 0.288 | |||
| Use of immunosuppressant | 0.529/0.211 | 1.333 | 0.190 | |||



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print