Introduction
Preintervention planning for CVS
Asymptomatic versus symptomatic CVS
Availability of other access options
Availability of local expertise and logistics for managing CVS
Endovascular intervention for CVS
Percutaneous angioplasty
Stents
1 Primary stent placement after PTA without waiting for recurrence
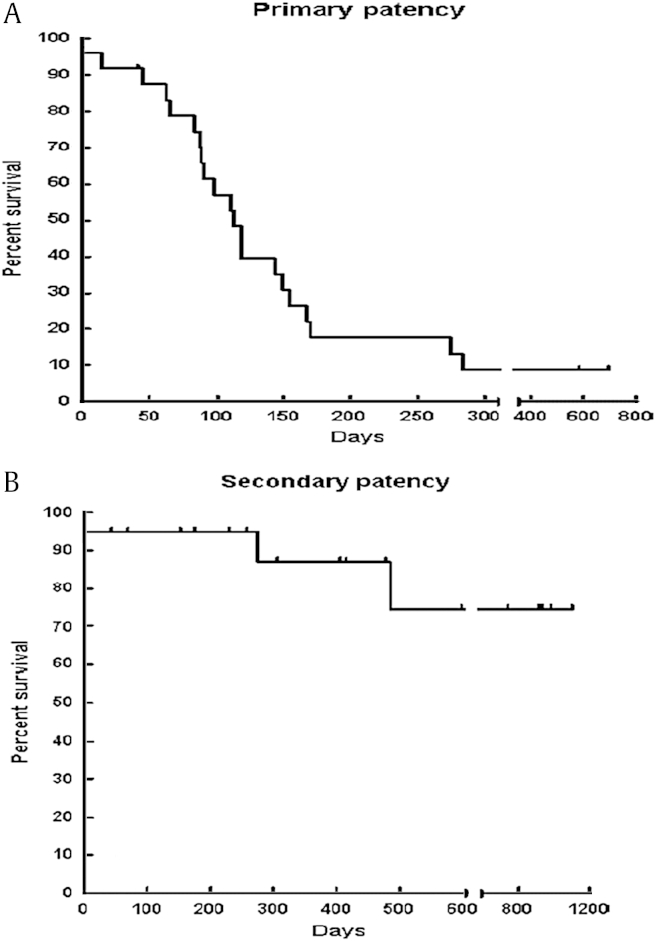
Primary angioplasty (PTA) with primary stent placement (PTS) has been considered in an attempt to improve short- and long-term results. The outcomes of primary PTA and PTS were compared at a tertiary referral academic medical center via a retrospective analysis of database of consecutive HD patients from 1995 to 2003 [11]. After primary high-pressure balloon angioplasty, stenting (PTS) was used for failed or suboptimal angioplasty in 26 patients with 26 CVS. PTA was done in 47 patients with 49 CVS. The stent group underwent 71 percutaneous interventions per stenosis (average, 2.7 ± 2.4 interventions), and the PTA group underwent 98 interventions per stenosis (average, 2.0 ± 1.6 interventions). Primary patency was equivalent between groups by Kaplan–Meier analysis, with 30-day rates of 76% for both groups and 12-month rates of 29% for PTA and 21% for PTS (P = 0.48). Assisted primary patency was also equivalent (P = 0.08), with a 30-day patency rate of 81% and 12-month rate of 73% for the PTA group versus PTS assisted patency rates of 84% at 30 days and 46% at 12 months. Ipsilateral HD access survival was equivalent between groups. The authors concluded that PTA or PTS for CVS was safe, with low rates of technical failure, required multiple additional interventions, and neither modality offered durable outcomes. Furthermore, PTS did not improve on the patency rates more than PTA and did not add to the longevity of ipsilateral HD access sites. However, the study displays the inherent dilemma of comparing patients who responded to PTA and those who did not and underwent stent placement for that reason. Inherent severity and nature of resistant lesions make the comparison of these populations difficult and unfair. It is likely that the lesions undergoing primary stenting would have suffered early occlusion without stenting because of suboptimal results, and the study could not provide a true comparison of primary angioplasty with primary stenting of lesions that actually were successfully treated with PTA alone.
2 Stent placement after failure or recurrence of CVS
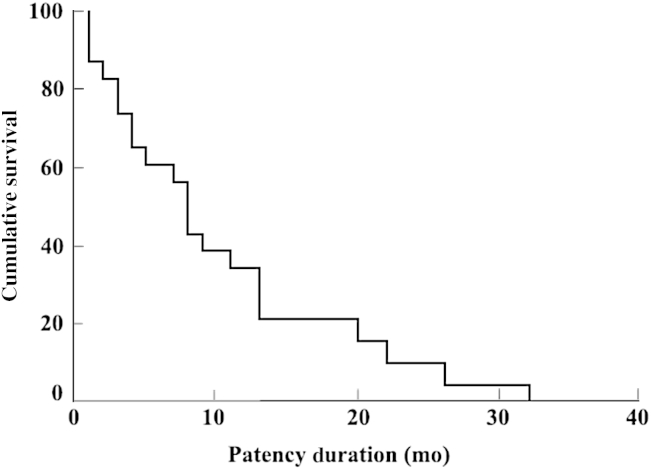
Stents are commonly used for difficult or failed lesions with angioplasty alone, and primary stenting is not common. The technical success rates for stents are usually quite good. However, the primary and secondary patency of stents is limited, probably because of the difficult and more severe or recurrent nature of the lesions requiring stent placement. Some initial studies showed promisingly good results with the use of stainless steel stents [15]. However, other studies showed only marginal results of such stents [16], [17]. A recent retrospective analysis of the nitinol shape memory alloy stents showed a significantly higher primary patency in central and peripheral veins though a systematic study of such stents has not been performed [18]. It is difficult to compare results of studies done over a period because stainless steel stents were used in earlier studies and more advanced stents were used in the later study, which may be responsible for better results. Irrespective of the primary technical success, repeated interventions can provide a significantly longer assisted patency. This may be appropriate in many clinical circumstances with end-stage vascular access.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















