Clinical utility of far-infrared therapy for improvement of vascular access blood flow and pain control in hemodialysis patients
Article information
Abstract
Background
Maintenance of a well-functioning vascular access and minimal needling pain are important goals for achieving adequate dialysis and improving the quality of life in hemodialysis (HD) patients. Far-infrared (FIR) therapy may improve endothelial function and increase access blood flow (Qa) and patency in HD patients. The aim of this study was to evaluate effects of FIR therapy on Qa and patency, and needling pain in HD patients.
Methods
This prospective clinical trial enrolled 25 outpatients who maintained HD with arteriovenous fistula. The other 25 patients were matched as control with age, sex, and diabetes. FIR therapy was administered for 40 minutes during HD 3 times/wk and continued for 12 months. The Qa was measured by the ultrasound dilution method, whereas pain was measured by a numeric rating scale at baseline, then once per month.
Results
One patient was transferred to another facility, and 7 patients stopped FIR therapy because of an increased body temperature and discomfort. FIR therapy improved the needling pain score from 4 to 2 after 1 year. FIR therapy increased the Qa by 3 months and maintained this change until 1 year, whereas control patients showed the decrease in Qa. The 1-year unassisted patency with FIR therapy was not significantly different from control.
Conclusion
FIR therapy improved needling pain. Although FIR therapy improved Qa, the unassisted patency was not different compared with the control. A larger and multicenter study is needed to evaluate the effect of FIR therapy.
Introduction
A well-functioning vascular access site and relief of needling pain are important factors for achieving adequate dialysis and improving the quality of life in hemodialysis (HD) patients. Although many surveillance methods have been developed to detect early vascular access dysfunction in patients with arteriovenous fistulas (AVFs), surveillance has not been shown to improve access survival in the grafts [1]. Thus, many researchers have tried to improve vascular access survival with medications and other techniques.
Far-infrared (FIR) radiation comprises electromagnetic waves with wavelengths that range from 5.6 to 1,000.0 μm. Infrared radiation transfers energy that is perceived as heat by thermoreceptors in the surrounding skin [2]. FIR therapy improves skin blood flow [3]. FIR therapy also improves endothelial function and reduces the frequency of some cardiovascular diseases [4], [5], [6]. Vascular access dysfunction is a major problem in HD patients. FIR radiation is a novel therapy that reported to improve the unassisted patency at 1 year in the patients with AVF population [7]. Lin et al [8] reported that FIR therapy improves the blood flow, maturation, and patency of a newly created AVF in patients with stage 4–5 chronic kidney disease. The aim of the present study was to evaluate the effects of FIR therapy on vascular access blood flow (Qa) and needling pain in HD patients.
Methods
Study design
This was a prospective clinical trial conducted from June 2013 to July 2014 at Soonchunhyang University Bucheon Hospital. All enrolled patients were maintained with 4 hours of HD 3 times/wk in our clinic. Eligible patients were those who either had no history of percutaneous transluminal angioplasty (PTA) or had undergone ≥1 PTA procedures, with the last PTA procedure successfully performed within 1 week before enrollment. We excluded patients who underwent HD treatment at a frequency other than 3 times/wk, had previously undergone FIR radiation therapy, had missed more than 10% of FIR treatments, had a history of kidney transplantation, were switched to peritoneal dialysis, or had severe disease with an estimated life expectancy of < 1 year. Data relevant to baseline characteristics [age, sex, HD therapy duration, PTA history, presence or absence of diabetes mellitus (DM), hypertension, liver disease, coronary artery disease, cerebrovascular disease, peripheral arterial disease, and medications] and patency outcomes were observed.
Patients provided written informed consent before beginning the study. The study was conducted in accordance with the Declaration of Helsinki and was approved by the Soonchunhyang University Bucheon Hospital Institutional Research Board.
FIR radiation therapy
FIR therapy was performed for 40 minutes during each HD session. We started FIR therapy within 30 minutes after starting HD. Specialized FIR emitters (WS TY-101N; WS Far Infrared Medical Technology Co., Ltd., Taipei, Taiwan) were used for FIR therapy. Electrified ceramic plates were positioned approximately 25 cm above the skin surface at the needling site. The irradiating power density was about 10 and 20 μW/cm2 when the radiator was set at a distance of 30 and 20 cm above the skin surface, respectively. Radiation therapy was continued in every HD session.
Measurement of Qa and pain
Qa was monthly measured within 2 hours after start of HD. Measurement was performed by the ultrasound dilution method using a Transonic HD03 HD monitor (Transonic Systems, Ithaca, NY, USA) [9]. Qa was measured before and after intervention in patients with FIR therapy. Needling pain and anxiety in patients were measured with a numeric rating scale. The scale indicated pain severity on a scale of 0–10 points, which correlated with no pain and uncontrolled severe pain, respectively.
The mean baseline Qa and pain were obtained during the 1 week before the start of the study. These parameters were then recorded at months 1, 3, 6, and 12 after beginning the study.
Study protocol
Effect of FIR therapy in a single HD session
We measured the study participants' Qa, body temperature (BT), and pain and anxiety scores before and after FIR therapy. The patients scored their own pain and anxiety. The nurse checked the patients' skin status in terms of inflammation, burning, or other abnormalities; sense of itching before needling; bleeding site during HD; and time required for hemostasis.
Effect of 1 year of FIR therapy on Qa and unassisted patency
We measured the study participants' Qa, BT, and pain and anxiety scores before and at months 1, 3, 6, and 12 after the start of the study. The primary outcome was the unassisted patency, which was defined as the time from study commencement to the first episode of AVF malfunction. We defined AVF malfunction as the need for any interventional procedure (surgery or angioplasty) to correct an occlusive or malfunctioning AVF that could not sustain an extracorporeal blood flow of > 200 mL/min during HD.
To analyze the effect of Qa and unassisted patency, we compared the study participants who underwent 1 year of FIR therapy with control patients who received no intervention for their access site during the study period. We matched the controls and participants by age, sex, and presence of DM.
Statistical analysis
Statistical differences in clinical characteristics and Qa per month between the 2 groups were evaluated using the χ2 test or Fisher's exact test for categorical variables and Student's t test or the Wilcoxon rank-sum test for numerical variables. Univariate and multivariate Cox proportional hazard regression analyses were conducted to determine the predictors of unassisted patency survival. The survival probability of unassisted patency was estimated according to the Kaplan–Meier method, and survival curves were compared with the log-rank test. The Wilcoxon signed-rank test was used to compare differences in Qa between pre- and post-FIR therapy. All statistical analyses were performed using SAS software (version 9.4 for Windows; SAS Institute, Inc., Cary, NC, USA). The statistical significance level was set at P < 0.05 (2-tailed).
Results
Twenty-five HD patients (mean age, 52.6 ± 10.7 years; 40% female) were enrolled in FIR therapy and other 25 HD patients were matched as control after the study. Table 1 compares the baseline characteristics between the FIR therapy group and control group. The clinical baseline characteristics were similar in both goups with the exception of treatment with calcium channel blockers and anticoagulation agents.
One patient transferred to another hospital. Seven patients (28%) refused to continue the FIR therapy. In total, 17 patients completed 1 year of FIR therapy. The follow-up duration was 9.8 ± 3.8 months. Thrombosis occurred in 2 patients at 0.5 and 5 months, respectively.
Effect of a single session of FIR therapy
We analyzed all FIR sessions of all participants to evaluate a single session of FIR therapy. The total number of sessions was 107. There was no significant difference in Qa, systolic blood pressure (BP), or diastolic BP before and after a single session of FIR therapy. The median BT increased from 36.6°C to 37.2°C after FIR therapy (P < 0.001; Table 2).
No patient experienced a burn episode during FIR therapy. Six patients complained itching sensations in 28 sessions. FIR therapy improved itching sensation in 11 sessions of them. Access site bleeding and delayed hemostasis at the needling sites after FIR therapy were seen in 3 and 19 sessions, respectively.
Effect of 1 year of FIR therapy
One and 6 patients stopped the FIR after 2 and 5 months, respectively, because of a high BT and discomfort. However, FIR therapy improved the needling pain scores from 4 to 2 (Table 3). The Qa of FIR therapy increased from 881.6 to 934.7 mL/min (P = 0.788). However, there was no statistical significance.
Effect of FIR therapy on Qa and survival
We compared the 1-year change in Qa between the study participants and control patients (Table 4). Because baseline Qa between the 2 groups was slightly different, we compared the change of Qa during follow-up months from baseline (Fig. 1). The change of Qa in patients with FIR therapy was increased by 3 months and maintained this change until 1 year, whereas that in controls was decreased. The 1-year unassisted patency rate of FIR therapy was not significantly different from that of controls (Fig. 2).
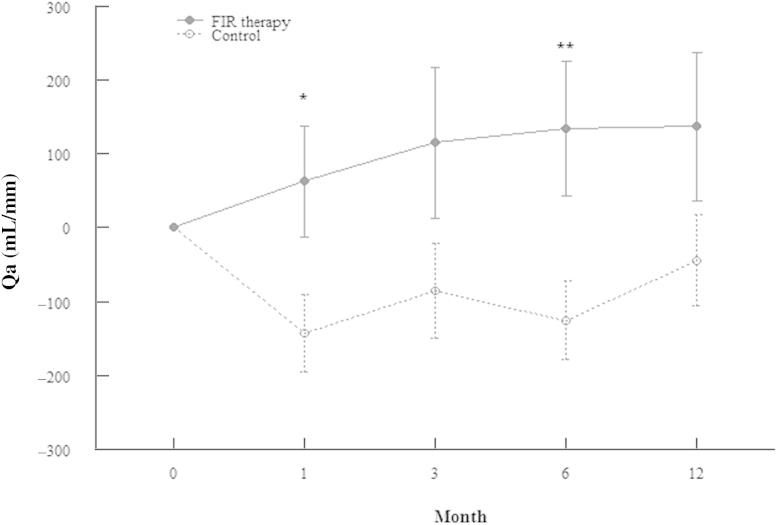
Comparison of change in Qa between FIR therapy group and control.
*P < 0.05, **P < 0.01 vs. control.
FIR, far-infrared; Qa, access blood flow.
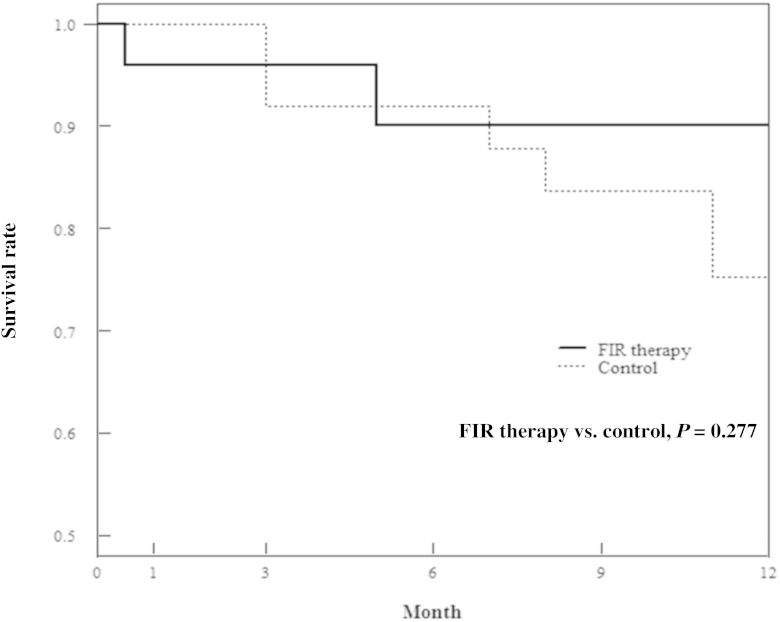
Comparison of the 1-year unassisted patency between FIR therapy and control group.
FIR, far-infrared.
Next, we analyzed the risk factors for the 1-year change of Qa (Table 5) and unassisted patency rate (Table 6). FIR therapy did not significantly influence the 1-year change of Qa in univariate analysis. Low initial blood flow rate was related with the 1-year change of Qa. The overall unassisted patency was fitted to a Cox proportional hazard regression model including age, sex, DM, hypertension, initial Qa, and FIR therapy in univariate and multivariate analyses. DM (hazard ratio, 5.61; P = 0.042) was only related with the 1-year unassisted patency in the multivariate Cox regression analysis.
Discussion
Patients on HD have numerous medical, social, and economic problems [10]. HD patients have a lower quality of life and more stress than patients with chronic kidney disease without dialysis [11]. Fear of needling and pain are major barriers to HD [12]. The buttonhole technique and topical anesthetic agents have been applied to vascular access to decrease needling pain [13], [14]. In the present study, FIR therapy decreased the needling pain score from 4 to 2 during 12 months (Table 3). This is similar to our previous study, which showed that the buttonhole technique decreased the needling pain score [15].
Our results also evaluated the safety of FIR therapy. Of the 25 patients, 17 (68%) continued FIR therapy. Our patients discontinued the intervention more frequently than in other reports (3.4–12.5%) [7], [8]. Although others [7], [16] reported no complications associated with FIR therapy, side effects such as an itching sensation and delayed hemostasis were seen in the present study. This result is in line with the previous report of Lin et al [17].
A single session of FIR increased the BT and tended to increase Qa and decrease systolic pressure (Table 2). Lin et al [7] did not observe on BT or BP but reported a significant incremental change in access flow with a single session of FIR therapy.
Fig. 1 showed that the FIR therapy increases Qa by 3 months and maintained until 1 year, whereas control patients had the decrease of Qa. This trend was revealed in a previous study, in which Lin et al [17] showed that FIR therapy improves access flow and maturation of newly created AVFs. Mean HD vintage of our patients was 68.3 months. In other words, FIR therapy can improve Qa in old access.
Although non-DM patients had the greater change of Qa in FIR therapy compared with DM patients, those with initial Qa < 500 mL/min had the greater change compared with those with initial Qa > 500 mL/min after 1 year of FIR therapy (data not shown).
We analyzed FIR therapy as a predictor of unassisted patency, adjusting for age, sex, DM, hypertension, and low initial Qa (Table 6). FIR therapy tended to improve the 1 year unassisted patency in multivariable analysis (hazard ratio = 0.09, P = 0.071). Lin et al [7] reported that the 1-year change in Qa and unassisted patency in 145 AVF patients were mean 36.3 ± 166.2 mL/min and 85.9%, respectively. Half (77 of 145) of the patients have received a PTA previously. Our 1-year change in Qa and unassisted patency in 25 AVF patients with FIR therapy were mean 137.6 ± 415.1 mL/min and 90%, respectively. Lai et al [16] reported that FIR therapy after PTA enhanced unassisted patency at 1 year in arteriovenous graft (AVG), whereas it had no effect in AVF.
Old age, female, and DM had no effect on the 1 year changes in Qa (Table 5). Patients with Qa < 500 mL/min at the baseline and those without hypertension had greater changes of Qa after 1 year. An initial Qa of < 500 mL/min is a known risk factor for poor primary patency [18]. Robbin et al [19] suggested that 2 risk factors for AVF maturation are a minimum vein diameter of < 4 mm and a Qa of < 500 mL/min. Lin et al [8] observed that FIR therapy improved physiological maturation (defined as an AVF diameter of ≥4 mm and Qa of ≥500 mL) at 3 months after AVF creation. In our results, initial Qa of < 500 mL/min did not influence the 1-year unassisted patency. If we could improve Qa at anytime, we can improve the access survival.
Until now, we do not have the definite therapy for prevention of vascular access failure. The surveillance and pre-emptive PTA were disappointed in this point. Although systemic management by agents such as dipyridamole plus aspirin and fish oil have been successful, systemic levels of such agents may expose the patients to significant adverse effects [20], [21]. Local therapies were therefore developed. FIR therapy is a perivascular approach, which is appealing given that (1) the adventitial and medial layers are thought to be the source of many cells that populate the neointimal hyperplasia and (2) denudation of the endothelial layer is avoided [22]. FIR therapy has thermal and nonthermal effects. Upregulation of endothelial nitric oxide synthase is considered to be a thermal effect [5], [23], [24]. Decreases in oxidative stress, suppression of inflammation, and improved endothelial function [5], [20], [24], [25], [26] have been suggested to be nonthermal effects. FIR therapy promotes microvascular angiogenesis, increases skin microcirculation, and improves skin wound healing [3], [26], [27]. Future studies should focus on these mechanisms in humans.
This single-center, clinical study has some limitations. First, it was not a placebo-controlled study. Since blinding regarding the intervention was impossible, we could not randomize patient with FIR therapy and control at initial study design. Although 25 AVF patients with FIR therapy were prospectively studied, the control patients were paired for the effect on Qa and patency after 1-year FIR therapy. Second, the enrolled patients were not homogeneous. They had varied PTA histories and comorbidities. The percentage of use of antihypertensive agents, especially calcium channel blocker, and anticoagulation agents in the FIR group was greater than that in the control group. Third, many patients dropped out. If we shorten FIR therapy during 3 months, our result should be more powerful. Fourth, there are no data on pain in the control group, and it is not clear whether the improvement of pain in the study group can be attributed solely to FIR therapy.
However, this study also has strengths. First, it evaluated the safety of FIR therapy. Second, it evaluated the effects of FIR therapy on needling pain. Third, we showed the 1-year effect of FIR therapy in Qa and patency. Fourth, we suggested a question about the duration of FIR therapy.
In conclusion, we have demonstrated that FIR therapy improves needling pain. Although FIR therapy improved Qa, FIR therapy did not improve unassisted 1-year patency compared with the control. However, more large and multicenter studies are needed to evaluate the effect of FIR therapy on access survival in AVF patients.
Conflicts of interest
All authors have no conflicts of interest to declare.
References
Acknowledgments
The authors thank Bora Lee and Jong Hye Kim for thoughtful consulting of the statistics and devotion to patient care, respectively.
This study was supported by the Soonchunhyang University Research Fund (20160000).
EH Cho and HM Jo underwent FIR therapy at AKU. C Min and YS Ji collected patients' data. MY Park and SD Hwang revised this manuscript. JK Kim reviewed and supervised this study.





