| Kidney Res Clin Pract > Volume 39(2); 2020 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (HI17C0530). The funders had no role in study design, data collection and analysis, decision to publish, or manuscript preparation. This study used the database from the NHIS of Korea. The approach was approved by the organization (NHIS-2017-1-346).
AuthorsŌĆÖ contributions
Sehoon Park, Hajeong Lee, Kwon Wook Joo, and Dong Ki Kim contributed to the conception and design of the study. Sehoon Park, Soojin Lee, Yaerim Kim, Yeonhee Lee, Min Woo Kang, Kyungdo Han, Jung Pyo Lee, Kwon Wook Joo, Chun Soo Lim, Yon Su Kim, and Dong Ki Kim provided statistical expertise and interpreted the data. Sehoon Park and Kyungdo Han performed the main statistical analysis, assisted by Soojin Lee. Hajeong Lee, Jung Pyo Lee, Kwon Wook Joo, Chun Soo Lim, and Yon Su Kim, and Dong Ki Kim offered advisement regarding the data interpretation. Dong Ki Kim obtained funding and supervised the overall project. All of the authors contributed to the manuscript. All of the authors reviewed the manuscript and approved the final version to be published.
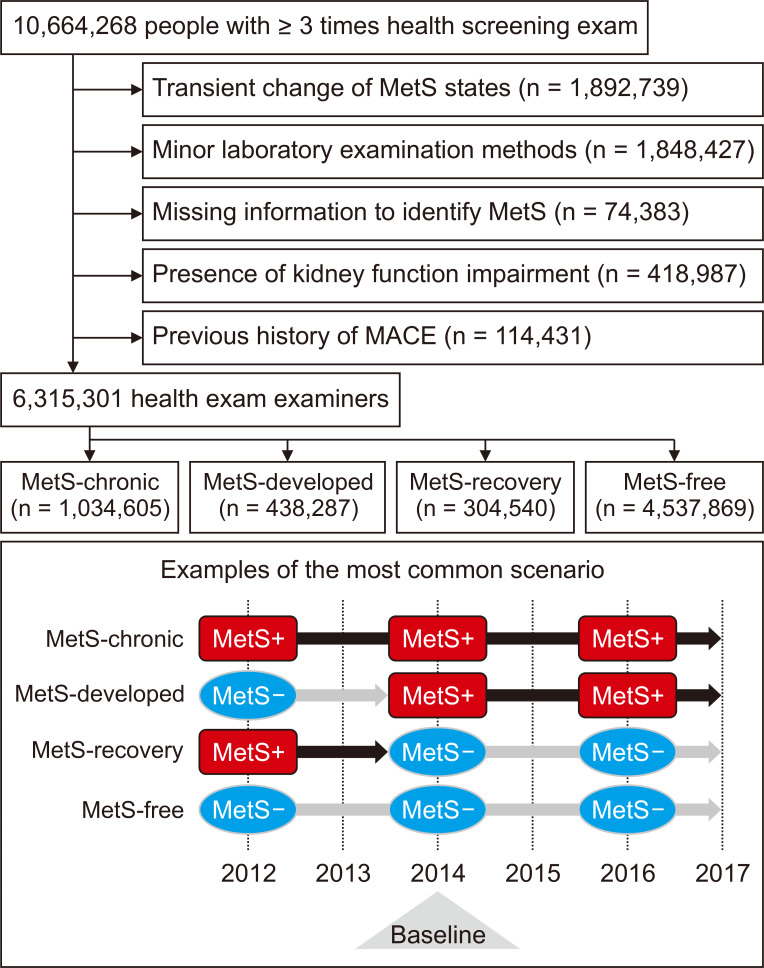
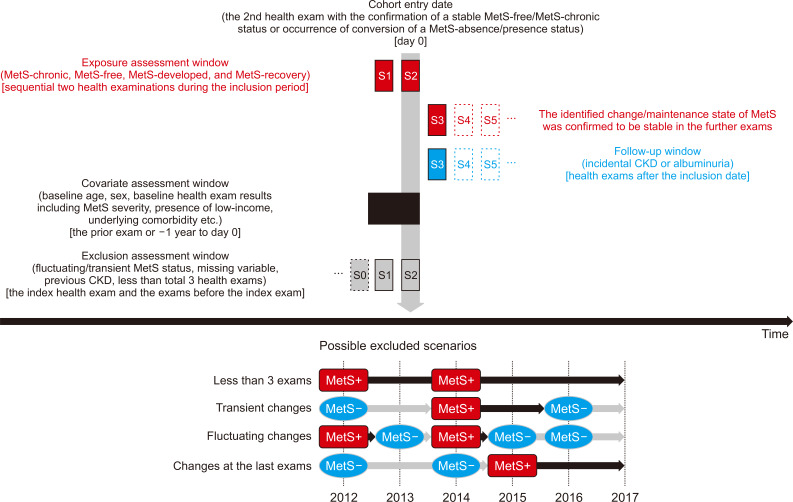
Figure┬Ā1
Study population criteria and representative scenarios.
Figure┬Ā2
Possible exclusion scenarios.
Figure┬Ā3
Risk of incidental chronic kidney disease (CKD) and albuminuria in the study groups.

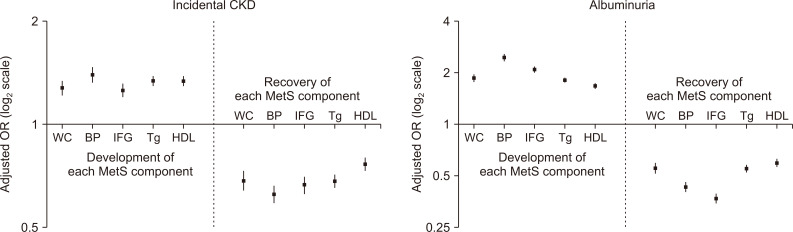
Figure┬Ā4
Changes in metabolic syndrome (MetS) components and their associations with the risks of the study outcomes.
Table┬Ā1
| Variablea | MetS-free (n = 4,537,869) | MetS-recovery (n = 304,540) | MetS-developed (n = 438,287) | MetS-chronic (n = 1,034,605) |
|---|---|---|---|---|
| Age (yr) | 43.5 ┬▒ 12.2 | 50.4 ┬▒ 12.1 | 51.9 ┬▒ 12.1 | 56.0 ┬▒ 11.6 |
| 20-39 | 1,783,874 (39.3) | 60,157 (19.8) | 74,950 (17.1) | 96,080 (9.3) |
| 40-59 | 2,282,702 (50.3) | 174,054 (57.2) | 243,800 (55.6) | 520,425 (50.3) |
| 60-79 | 458,331 (10.1) | 68,094 (22.4) | 116,330 (26.5) | 405,349 (39.2) |
| Ōēź 80 | 12,962 (0.3) | 2,235 (0.7) | 3,207 (0.7) | 12,751 (1.2) |
| Sex (male) | 2,460,443 (54.2) | 197,954 (65.0) | 275,926 (63.0) | 588,731 (56.9) |
| BMI (kg/m2) | 22.5 ┬▒ 2.7 | 24.6 ┬▒ 2.9 | 25.7 ┬▒ 3.1 | 26.5 ┬▒ 3.3 |
| Low income statusb | 743,447 (16.4) | 55,003 (18.1) | 80,770 (18.4) | 217,976 (21.1) |
| MetS components | ||||
| Waist circumference (cm) | 76.5 ┬▒ 7.9 | 82.4 ┬▒ 7.6 | 86.3 ┬▒ 8.0 | 88.2 ┬▒ 8.4 |
| Systolic BP (mmHg) | 116.9 ┬▒ 12.5 | 123.1 ┬▒ 12.8 | 128.8 ┬▒ 13.3 | 129.87 ┬▒ 14.0 |
| Diastolic BP (mmHg) | 73.3 ┬▒ 8.8 | 77.1 ┬▒ 9.0 | 80.4 ┬▒ 9.5 | 80.3 ┬▒ 9.8 |
| Glucose (mg/dL) | 91.9 ┬▒ 13.01 | 98.4 ┬▒ 21.0 | 106.3 ┬▒ 23.9 | 115.7 ┬▒ 33.7 |
| Tg (mg/dL) | 98.7 ┬▒ 61.2 | 135.7 ┬▒ 87.6 | 191.2 ┬▒ 135.2 | 200.4 ┬▒ 149.8 |
| HDL (mg/dL) | 59.4 ┬▒ 14.5 | 53.1 ┬▒ 13.5 | 49.6 ┬▒ 13.7 | 48.6 ┬▒ 13.4 |
| CCI score | 0.5 ┬▒ 0.9 | 0.8 ┬▒ 1.1 | 1.0 ┬▒ 1.3 | 1.6 ┬▒ 1.6 |
| Baseline lab parameters | ||||
| Hemoglobin (g/dL) | 14.1 ┬▒ 1.6 | 14.4 ┬▒ 1.6 | 14.5 ┬▒ 1.6 | 14.3 ┬▒ 1.6 |
| AST (IU/L) | 23.3 ┬▒ 15.8 | 26.0 ┬▒ 19.2 | 28.7 ┬▒ 20.3 | 29.6 ┬▒ 22.0 |
| ALP (IU/L) | 21.3 ┬▒ 20.1 | 26.8 ┬▒ 24.0 | 32.9 ┬▒ 27.0 | 33.7 ┬▒ 28.4 |
| Cr (mg/dL) | 0.87 ┬▒ 0.26 | 0.89 ┬▒ 0.26 | 0.89 ┬▒ 0.25 | 0.88 ┬▒ 0.29 |
| eGFR (mL/min/1.73 m2) | 93.9 ┬▒ 24.5 | 91.2 ┬▒ 23.9 | 90.0 ┬▒ 21.9 | 88.9 ┬▒ 23.0 |
| Urine albuminuria (Ōēź 1+) | 50,051 (1.1) | 4,501 (1.5) | 9,034 (2.1) | 35,361 (3.4) |
Table┬Ā2
| Model 1 (number of present MetS components adjusted)a | Model 2 (measured values of MetS criteria adjusted)b | |||||||
|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||
| Albuminuria | Incidental CKD | Albuminuria | Incidental CKD | |||||
|
|
|
|
|
|||||
| Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Intergroup analysis with adjustment of previous MetS severityc | ||||||||
| Recovery from MetS (ref. = MetS-chronic group) | 0.56 (0.54-0.58) | < 0.001 | 0.74 (0.72-0.77) | < 0.001 | 0.58 (0.56-0.60) | < 0.001 | 0.74 (0.72-0.76) | < 0.001 |
| Development of MetS (ref. = MetS-free group) | 1.58 (1.54-1.62) | < 0.001 | 1.27 (1.24-1.30) | < 0.001 | 1.54 (1.50-1.58) | < 0.001 | 1.26 (1.23-1.29) | < 0.001 |
| Intergroup analysis with adjustment of baseline MetS severityd | ||||||||
| Previous history of MetS (MetS-recovery, ref. = MetS-free) | 1.16 (1.13-1.20) | < 0.001 | 1.13 (1.10-1.17) | < 0.001 | 1.15 (1.11-1.18) | < 0.001 | 1.11 (1.08-1.15) | < 0.001 |
| Previously free from MetS (MetS-developed, ref. = MetS-chronic) | 0.73 (0.72-0.75) | < 0.001 | 0.84 (0.82-0.86) | < 0.001 | 0.77 (0.75-0.78) | < 0.001 | 0.84 (0.82-0.86) | < 0.001 |
The base multivariable model included the following variables at the inclusion date: age, sex, low income, Charlson comorbidity index scores, body mass index, and baseline laboratory parameters, including estimated glomerular filtration rate, aspartate aminotransferase/alanine aminotransferase, hemoglobin, and the presence of dipstick albuminuria.
a In addition to the variables, the number of present MetS components was adjusted in model 1. bThe model was same as the model 1 except that actual measured parameters of MetS criteria being included in the model instead the number of present components. cIn intergroup analysis 1, the previous MetS parameters were adjusted, as the study groups had same MetS presence/absence state in the previous period. dIn intergroup analysis 2, the baseline MetS parameters were adjusted, as the MetS presence/absence state was the same during the follow-up period.
Table┬Ā3
| Model 1a | Model 2b | |||
|---|---|---|---|---|
|
|
|
|||
| Adjusted HR (95% CI) | P value | Adjusted HR (95% CI) | P value | |
| Incidental CKD | ||||
| Developed components (in MetS-developed vs. MetS-free) | ||||
| Central obesity | 1.23 (1.17-1.29) | <0.001 | 1.24 (1.18-1.31) | <0.001 |
| High blood pressure | 1.36 (1.29-1.44) | <0.001 | 1.37 (1.29-1.44) | <0.001 |
| Impaired fasting glucose | 1.20 (1.15-1.26) | <0.001 | 1.25 (1.19-1.31) | <0.001 |
| Dyslipidemia, Tg | 1.30 (1.25-1.34) | <0.001 | 1.27 (1.22-1.31) | <0.001 |
| Dyslipidemia, HDL | 1.29 (1.25-1.34) | <0.001 | 1.28 (1.24-1.33) | <0.001 |
| Recovered components (in MetS-recovery vs. MetS-chronic) | ||||
| Central obesity | 0.73 (0.69-0.78) | <0.001 | 0.71 (0.67-0.76) | <0.001 |
| High blood pressure | 0.65 (0.61-0.69) | <0.001 | 0.65 (0.61-0.69) | <0.001 |
| Impaired fasting glucose | 0.71 (0.67-0.75) | <0.001 | 0.73 (0.68-0.770) | <0.001 |
| Dyslipidemia, Tg | 0.71 (0.68-0.74) | <0.001 | 0.71 (0.68-0.74) | <0.001 |
| Dyslipidemia, HDL | 0.81 (0.77-0.84) | <0.001 | 0.78 (0.75-0.82) | <0.001 |
| Albuminuria | ||||
| Developed components (in MetS-developed vs. MetS-free) | ||||
| Central obesity | 1.73 (1.65-1.83) | <0.001 | 1.72 (1.63-1.81) | <0.001 |
| High blood pressure | 2.34 (2.22-2.46) | <0.001 | 2.24 (2.13-2.36) | <0.001 |
| Impaired fasting glucose | 1.98 (1.89-2.07) | <0.001 | 2.03 (1.94-2.13) | <0.001 |
| Dyslipidemia, Tg | 1.68 (1.62-1.74) | <0.001 | 1.62 (1.56-1.68) | <0.001 |
| Dyslipidemia, HDL | 1.54 (1.49-1.60) | <0.001 | 1.49 (1.43-1.55) | <0.001 |
| Recovered components (in MetS-recovery vs. MetS-chronic) | ||||
| Central obesity | 0.60 (0.56-0.65) | <0.001 | 0.60 (0.56-0.64) | <0.001 |
| High blood pressure | 0.46 (0.43-0.49) | <0.001 | 0.47 (0.44-0.50) | <0.001 |
| Impaired fasting glucose | 0.39 (0.36-0.42) | <0.001 | 0.44 (0.41-0.47) | <0.001 |
| Dyslipidemia, Tg | 0.59 (0.56-0.62) | <0.001 | 0.60 (0.57-0.63) | <0.001 |
| Dyslipidemia, HDL | 0.49 (0.45-0.53) | <0.001 | 0.65 (0.62-0.69) | <0.001 |
CI, confidence interval; CKD, chronic kidney disease; HDL, high-density lipoprotein; HR, hazard ratio; MetS, metabolic syndrome; Tg, triglycerides.
The base multivariable model included the following variables at the inclusion date: age, sex, low income, Charlson comorbidity index scores, body mass index, and baseline laboratory parameters, including estimated glomerular filtration rate, aspartate aminotransferase/alanine aminotransferase, hemoglobin, and the presence of dipstick albuminuria.
a In addition to the variables included in the base model, the number of present MetS components in the health exam just before the inclusion was adjusted in the model 1. bThe model was same as the model 1 except for the actual measured parameters of MetS criteria being included in the model instead of the number of present components.




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print