| Kidney Res Clin Pract > Volume 39(4); 2020 > Article |
|
Abstract
Background
There is a paucity of data on long term-outcomes of children who undergo acute peritoneal dialysis (PD) in resource-limited settings. We reviewed the outcomes of children who underwent PD after 18 months of follow-up.
Methods
We conducted a prospective cohort study in children with acute kidney injury (AKI) who underwent PD. Diagnosis of AKI was based on the 2012 Kidney Disease Improving Global Outcomes definition. We assessed outcomes of in-hospital mortality, 18-month post-dialysis survival, factors associated with survival, and progression to chronic kidney disease (CKD).
Results
Twenty-nine children with a median age of 6 (3 to 11) years underwent acute PD. In-hospital mortality was 3/29 (10.3%) and rose to 27.6% during follow-up. Seven (24.1%) children were lost to follow-up. Of the 14 remaining children, six (42.9%) experienced full recovery of renal function, while eight (57.1%) progressed to CKD. Among those who experienced full recovery, median (interquartile range) estimated glomerular filtration rate (eGFR) rose from 12.67 (7.05, 22.85) mL/min/1.73 m2 to 95.56 (64.50, 198.00) mL/min/1.73 m2, P = 0.031. No significant changes in median eGFR from baseline were observed among those who progressed to CKD (P = 0.383) or in non-survivors (P = 0.838). According to Kaplan-Meier curve analyses, 18-month survival during follow-up was 66.0% (95% CI, 45.0% to 86.5%). Age < 5 was associated with greater likelihood of survival (OR, 3.217; 95% CI, 1.240 to 8.342).
Acute kidney injury (AKI) is a sudden decline in renal function that is characterized by retention of nitrogenous waste products [1]. Globally, it is one of the leading causes of childhood death, with varying incidence, etiologies, and outcomes [2]. The pooled global incidence of pediatric AKI is estimated at 33.7% (95% confidence interval [CI], 26.9 to 41.3) [3]. However, the incidence of pediatric AKI varies from less than 1% to as high as 60% depending on the population [4,5], and low middle-income countries (LMIC) have higher prevalence and poorer outcomes compared with high-income countries [6,-8]. A number of factors underlies the high morbidity and mortality of pediatric AKI in LMIC, including the predominance of infection-related causes, delayed presentation, inadequate access to specialist care, lack of access to renal replacement therapy (RRT), and paucity of data, especially from Africa. When data are available, they are mainly from tertiary health facilities that treat relatively few patients [7,-10].
Despite the challenges in caring for children with AKI in developing countries, there has been recent progress in some tertiary health facilities in LMIC that now offer acute peritoneal dialysis (PD) using adaptable techniques [11,-13]. This pragmatic approach utilizes improvised PD catheters and locally constituted PD fluids. PD exchange procedures are performed manually at bedside [14]. There are some data regarding treatment and outcomes of children with AKI and acute PD in LMIC, especially Nigeria [15,-17]. However, these studies are limited to analyses of procedure complications and in-hospital mortality. Very few studies include outcomes measured as long as three months after onset of AKI [12,17]. Thus, the long term-outcomes of children who undergo acute PD for AKI in developing countries remain largely unknown. Therefore, in this study, we determined the outcomes of children with acute PD for AKI who were treated at a faith-based hospital in southwest Nigeria. The outcomes assessed were in-hospital mortality, progression to chronic kidney disease (CKD), mortality rate on follow-up, and factors associated with survival.
We conducted this study at Bowen University Teaching Hospital (BUTH) Ogbomosho, southwest Nigeria, which is a faith-based teaching hospital. The BUTH Research Ethics Review Committee provided ethical approval for the study (ethical approval number: NHERC/12/04/2012). In addition, we obtained consent from the parents/caregivers and assent from older children.
This was a prospective cohort study that involved 29 pediatric patients who underwent acute PD out of 107 with AKI managed at BUTH between December 2014 and January 2017. We included children aged 18 years and younger and excluded children who were treated with hemodialysis (HD) and conservative management for AKI from the final data analysis. Children with end stage renal disease that required continuous renal replacement therapy were also excluded. All patients with AKI were admitted to the emergency pediatric unit of the hospital. A Microsoft Excel spreadsheet was used to capture data for children who had AKI including acute PD during hospital admission. The spreadsheet captured detailed history and physical examination data for each child at admission. Anthropometric data including weight, height, body mass index, and body surface area were also documented for each patient. Upon admission, data for complete blood count, peripheral blood film appearance, blood film for the malaria parasite, urinalysis and urine microscopy, electrolytes, urea, creatinine, calcium, phosphate, and abdominal ultrasound were obtained for all patients. We also recorded information for blood cultures, hemoglobin genotyping, bleeding time, prothrombin time, activated partial thromboplastin time, renal biopsy (where indicated), complement C3 and C4, anti-double stranded DNA, and antinuclear antibody test for some patients as clinically indicated. For the recruited children, serum creatinine, urea, and electrolyte studies were repeated, and clinical details were updated upon follow-up. Estimated glomerular filtration was calculated using the original Schwartz formula (estimated glomerular filtration rate, eGFR = k × L/Scr, mL/min/1.73 m2, L was height in c, Scr was serum creatinine mg/dL, and k was the constant) [18]. The values of K used were 0.45 for infants, 0.55 for children and females, and 0.7 for males’ adolescents.
The indications for RRT included the presence of one or more of the following features: uremic syndrome, poor response to medical treatment for severe or rapidly rising azotemia, hyperkalemia (serum potassium > 6.5 mmol/L), severe metabolic acidosis (serum bicarbonate less than 10 mmol/L), persistent oligoanuria, un-affordability of HD, and other indications according to the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. The indicating preference for PD over HD included hemodynamically unstable patients, weight less than 10 kg (only F-series dialyser cube; SA of 0.2 m2 was available for pediatric patients at the dialysis unit), and anticoagulant intolerance.
We carried out PD in a dedicated side room in the pediatric ward. All children had a urethral catheter passed for monitoring of urine output. Strict aseptic pre-conditions were maintained throughout the procedure. The head of the nephrology team, who is one of the authors, prescribed PD and inserted the catheter at bedside. Trained nurses carried out PD cycling manually with support of the resident physicians. We administered intravenous 2 mg/kg of intravenous ethamsylate 30 minutes before PD catheter insertion. The anterior abdomen was cleaned with antiseptic (povidone-iodine), and the surgical site was infiltrated with 0.5% lidocaine at 4 mg/kg. A 0.5 cm incision was made about 2.5 cm below the umbilicus with a size 15 surgical blade deep through to the fascia. If a sterile, reusable stylet was not available, an adapted, sterile bicycle spoke was inserted into a fenestrated size 10 feeding tube for resilience through the skin nick with sustained pressure until we felt some “give.” The adapted stylet was removed, and the tube was gently advanced into the peritoneal cavity.
A deep subcutaneous purse-string suture was inserted if the catheter ran loosely through the incision. The improvised PD catheter was connected to a 3-way tap, with the second end connected to the PD fluid tubing, while the third drained the effluent into an improvised urinary bag. We used standard PD fluid (Fresenius SE & Co., Bad Homburg, Germany) with 1.5% glucose containing Na+ 134 mmol/L, Ca2+ 1.75 mmol/L, Mg2+ 0.5 mmol/L, Cl- 103.5 mmol/L, pH 5.5, and caloric density of 60 kcal/L for PD. If a patient was edematous, 2.3% glucose was alternated with 1.5% dextrose PD fluids. We spiked the PD with 100 IU/L of heparin and 4 mg/L of gentamycin. We ran 5 to 10 mL/kg PD fluid for the first three cycles without dwell time to ensure smooth operation. Subsequently, the PD fluid was increased to 20 to 40 mL/kg depending on tolerability and absence of respiratory distress.
Cycles of PD were performed until patients regained full consciousness, urinary output greater than 1.5 mL/kg/hr, and were ambulating. However, in the event of recurrence of oliguric, anuric, or rising azotemia or worsening encephalopathy, dialysis was re-commenced. If potassium was less than 4 mmol/L, a potassium supplement of 4 mmol/L was added to the PD fluid. Patients’ vital signs were closely monitored throughout the procedures. The presence of abdominal pain, tenderness, guarding, and cloud effluent of PD fluid were suggestive of peritonitis. At the end of the procedure, the catheter tip and PD fluid were sent for microscopy culture and sensitivity when indicated. Electrolyte urea and creatinine levels and clinical state were monitored until discharge.
AKI was defined as an at least 0.3 mg/dL rise in baseline serum creatinine within 48 hours, 1.5 times increase in baseline creatinine within seven days, or urine output ≤ 0.5 mL/kg/hr within six hours based on the 2012 KDIGO criteria [19]. We obtained baseline creatinine values and estimated the eGFR at the first point of contact during admission. CKD was defined as eGFR less than 60 mL/min per 1.73 m2 at least three months after discharge [20,21]. Anuria was defined as urine < 1 mL/kg/d [22].
A child was defined as “lost to follow-up” when he or she missed two consecutive clinic visits and attempts to communicate with the parents by phone failed. Post-hospitalization mortality was confirmed by follow-up calls to caregivers/parents following two consecutive clinic defaults. Five children with nephrotic syndrome and AKI received angiotensin-converting enzyme inhibitor therapy as part of the management protocol during follow-up.
The primary outcome was 18-month survival after acute PD. The secondary outcomes were in-hospital deaths, progression to CKD (defined as eGFR less than 60 ml/min per 1.73 m2 at least three months after discharge) [20], and factors associated with 18-month survival.
Data collected were analyzed using IBM SPSS version 23.0 for Windows (IBM Corp., Armonk, NY, USA). Means and standard deviations were used to summarize parametric continuous variables that were normally distributed (blood pressure). Age, weight, height, and body surface area were not normally distributed and were summarized as median with interquartile range (IQR). Discrete variables were summarized as frequency and percentage. The adjusted odds ratio (OR) was used to evaluate independent predictors of survival among patients (14 survivors and 8 non-survivors) with complete data at the end of follow-up. The variables were age, weight, height, body surface area, etiology, anuria, hypertension, duration of hospitalization, and number of dialysis sessions. The outcome of interest for the adjusted OR was survival at 18 months, which was plotted as a Kaplan-Meier curve. For plotting the curves, the primary event was 18-month survival of the cohort, and we censored those who were lost to follow-up and who survived beyond 18 months. The level of statistical significance was set at P < 0.05.
A total of 107 children aged 4 months to 18 years experienced AKI over the study period, for a cumulative incidence of 8.43 per 100,000 person-years. Of 107 children who had AKI, 73 (68.2%) were conservatively managed, 29 (27.1%) underwent PD, and 5 (4.7%) underwent HD, as shown in Fig. 1. The median (IQR) age of 29 children with acute PD was 6 (3 to 11) years, and most were male (n = 20, 69.0%). Fever was the most common clinical feature at presentation (n = 23, 79.3%), while six children (20.7%) had hypertension. We noted that 27 of 29 cases (93.1%) were community-acquired AKI, and 15 (51.7%) children had the non-oliguric form of AKI. The duration of PD ranged from 1 to 8 days, as shown in Table 1.
The most common cause of AKI was septicemia (n = 11, 37.9%). Other causes of AKI included malaria (n = 3), obstructive uropathy (posterior urethral valve) (n = 4), nephrotic syndrome (n = 5, 17.2%), acute glomerulonephritis (n = 3, 10.3%), and hemolytic uremic syndrome (n = 1, 3.4%), as shown in Table 2. The etiology of AKI was comparable among patients who underwent conservative management and those that received PD. Based on the index of severity (KDIGO AKI classification), 63.0% (46/73) of children in the conservative management group were stage 1, while 26.0% (19/73) and 11.0% (8/73) were stages 2 and 3, respectively (Table 2). The children in the PD group were all stage 3, as shown in Table 2. Mortality was significantly higher in children who underwent PD compared with children who were treated by conservative management (P = 0.021). The indications for PD were azotemia (n = 15, 51.7%), encephalopathy (n = 15, 51.7%), hyperkalemia (n = 3, 10.3%), and anuria (n = 9, 31.0%), and eight children had multiple indications (Table 3).
Of the 29 children who underwent acute PD, three died during hospitalization, yielding a case fatality rate of 10.3% (95% CI, 2.2% to 27.3%). The three patients who died during hospitalization were lost due to multiple organ dysfunction syndrome, as shown in Table 4. Five additional deaths were recorded during follow-up of the 26 survivors who had acute PD, yielding a cumulative mortality rate of 27.6% (95% CI, 12.7% to 47.2%) as shown in Fig. 1. During follow-up, two children died from febrile illness unrelated to kidney disease, one died from end stage kidney disease, and we were unable to obtain the details regarding cause of death in the remaining two children (Table 4). The Kaplan-Meier curve showed that the 18-month survival during follow-up was 66.0% (95% CI, 45.0% to 86.5%) as shown in Fig. 2. The mean survival was 18.6 months (95% CI, 14.8 to 22.4). Seven children (26.9%) were lost to follow-up. Of the 14 who survived to 18 months post dialysis, six (42.9%) had fully recovered renal function, while eight (57.1%) progressed to CKD as shown in Fig. 1. At the end of 18 months, among those who had full recovery, the median (IQR) eGFR rose from 12.67 (7.05, 22.85) mL/min/1.73 m2 baseline at admission to 95.56 (64.50, 198.00) mL/min/1.73 m2, P = 0.031. There was no significant increase in median eGFR from baseline among children who progressed to CKD (19.02 [10.15, 46.52] vs. 11.78 [6.80, 32.23] mL/min/1.73 m2, P = 0.383). Similarly, there was no significant change in eGFR among those who died during the study period (5.93 [4.47, 7.32] vs. 5.39 [4.99, 7.58] mL/min/1.73 m2, P = 0.838), as shown in Fig. 3.
Anthropometric indices (weight, height, and body surface area) were not associated with survival. Similarly, presence of sepsis (an etiology of AKI), hypertension, number of sessions of PD, and duration of hospitalization were not associated with increased survival. However, children younger than five years had better survival compared with those aged five years and above (OR, 3.217, 95% CI, 1.240 to 8.342) as shown in Table 5.
Acute PD remains the modality of choice for children with AKI who require RRT in LMIC and has variable outcomes. In the present study, 57.1% of survivors who underwent acute PD for AKI progressed to CKD within 18 months of dialysis. The progression to CKD observed in this study was higher than the 17% reported in a sample of Indian children, which may be partly explained by the shorter follow-up period of the previous study (three months) [23]. Similarly, the prevalence of CKD in this cohort was also higher than the 22.7% reported in Sudan among children who underwent PD for AKI [12]. The paucity of literature on long-term follow-up in children after acute PD for AKI limited further comparisons. Among adult survivors in the United States, 80% progress to CKD 30 day after dialysis for AKI [24]. The observation that more than half of the children in this present study developed CKD within 18 months following discharge suggests faster progression to CKD. This faster progression to CKD may be partly genetic, as progression of renal impairment in patients of African descent tends to differ from that in patients of western Eurasian descent [25]. It is also possible that the pathologic mechanisms of renal injuries in patients, such as oxidative damage, irreversible reduction in peritubular capillaries, and persistence of the inflammatory response post-dialysis despite improvement in GFR, may be responsible for poor outcomes [26,27].
This study revealed a very low in-hospital mortality rate of 10.3%, which rose cumulatively to 27.6% at follow-up. The in-hospital mortality rate observed in the present study is lower compared to the 30% to 41.2% previously observed among Nigerian children with acute PD for AKI [11,28]. The in-hospital mortality of 10.3% obtained in this study is lower than the 14.8% (in children that had PD without complication) and 21.4% (in children that had PD with complications) following post-cardiac surgery AKI in Denmark [29]. In contrast, the value obtained in this study was higher than the 5.6% among children who had undergone PD in India [30]. There are plausible reasons for the relatively low in-hospital mortality rate observed in this study, which include lower rate of infection, use of manufactured dialysate rather than constituted fluids, and early commencement of PD. Although there is a dearth of data to evaluate mortality during follow-up in children post-acute PD for AKI in developing countries, the cumulative mortality rate (27.6%) is lower than the 37.8% described among a cohort of adults who underwent HD dialysis for AKI [24].
In this study, presence of anuria during admission was not associated with increased mortality. This result differs from some earlier studies of children with AKI [13,30]. A retrospective study in India that found that the presence of anuria, septicemia, and severe infectious complications tend to be associated with increased mortality [13]. Similarly, in Brazil, presence of oligoanuria was associated with poor outcomes in children with AKI and sepsis [30].
Although we found no dissimilarities in baseline anthropometric indices of height, weight, and body surface area between survivors and non-survivors of AKI post-acute PD, a study in Denmark observed such a contrast. In that study, patient weight lower than 5 kg was a risk factor for increased mortality in children who required PD for AKI [29]. The inability to detect any differences in anthropometric indices between survivors and non-survivors may be related to the small sample size of this study. Malnutrition is associated with endothelial dysfunction, oxidative stress, high levels of proinflammatory cytokines such as soluble intercellular adhesion molecule-1, and elevated c-reactive protein, which may contribute to the higher mortality rate in children [31,-33].
This present study indicates that children younger than five years have better odds of survival than children five years and older. Although there is a paucity of comparable studies, two studies in India examined factors associated with mortality in children who underwent PD for AKI. They found no relationships with age [13,34]. However, a review of the long-term outcomes of chronic dialysis in children detected higher mortality in the younger age group [35]. Our observation of better survival among children under five may be related to treatable infection-related causes of AKI compared with the older patients who may develop AKI on background CKD with further risk of progression.
The etiologies of AKI observed in this study are consistent with the results of other studies from LMIC, in which infectious and congenital anomalies of the kidney and urinary tract (CAKUT) remain the leading causes of AKI [11,12]. The importance of this finding is that preventive nephrology in developing countries should focus on antenatal screening for CAKUT and prompt and appropriate management of common childhood infectious diseases. A high index of suspicion should be placed on children with urinary tract infection, as 13% of children who had PD for AKI in this present study had some form of obstructive uropathy as the underlying cause.
Although our study appears to be the first to follow a cohort of children who underwent acute PD for an extended period of 18 months in sub-Saharan Africa, there are some limitations. First, our sample size was relatively small, which may affect the statistical power of the study. Secondly, one-quarter of the children (n = 7, 26.9%) were lost to follow-up. Finally, no detailed autopsies were carried out to ascertain the cause of death for those who died during follow-up. Rather, we relied on information provided by their parents.
In conclusion, we observed faster progression to CKD among survivors of AKI who underwent acute PD than among those who did not. Progression of post-PD AKI to CKD occurred in more than half of the survivors. Age less than five years was associated with greater survival.
Notes
Authors’ contributions
Michael Abel Alao, Olayinka Rasheed Ibrahim, and Adanze Onyenonachi Asinobi conceptualized, analysed, drafted the initial manuscript, and revised the manuscript. Adanze Onyenonachi Asinobi and Akinwale Akinsola critically appraised and revised the manuscript. All authors read and approved the final manuscript.
Figure 1
Flowchart showing the cohort of children included in the study and outcomes during follow-up.
CI, confidence interval; eGFR, estimated glomerular filtration rate.
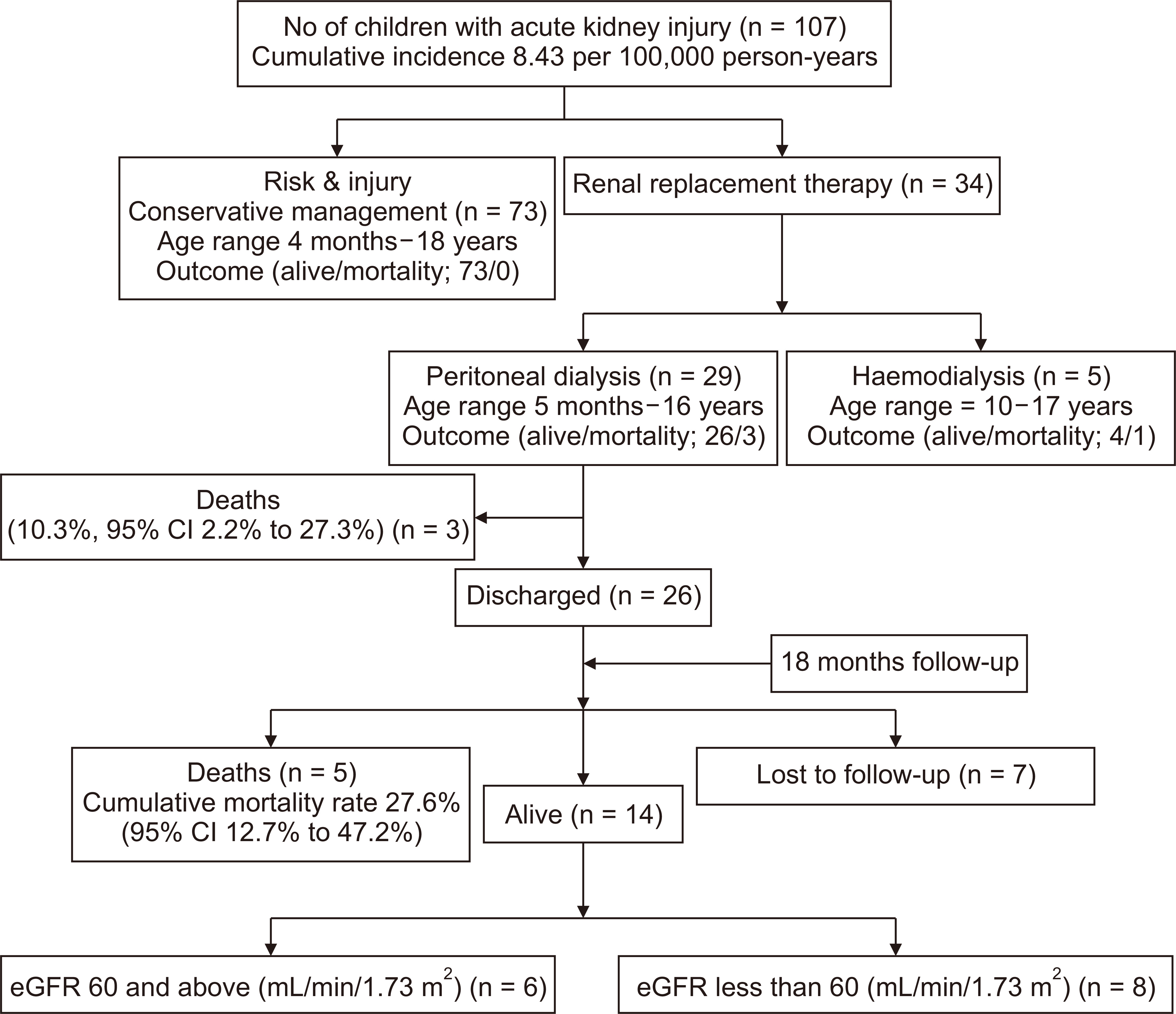
Figure 2
Eighteen-month survival among children who underwent acute peritoneal dialysis.
CI, confidence interval.
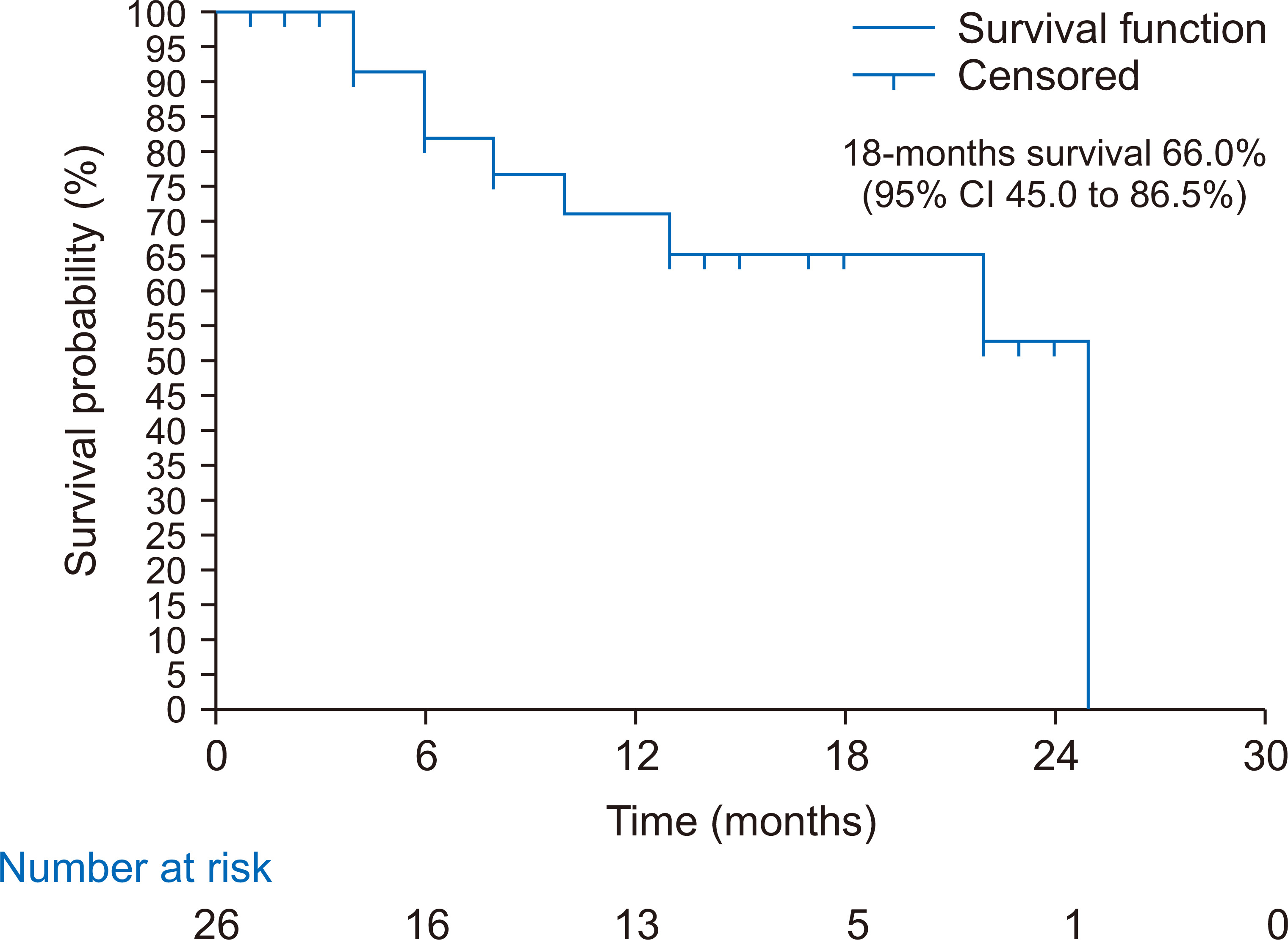
Figure 3
Estimated glomerular filtration rate (eGFR) at baseline (before peritoneal dialysis [PD]), after PD, and at follow-up.
CKD, chronic kidney disease.
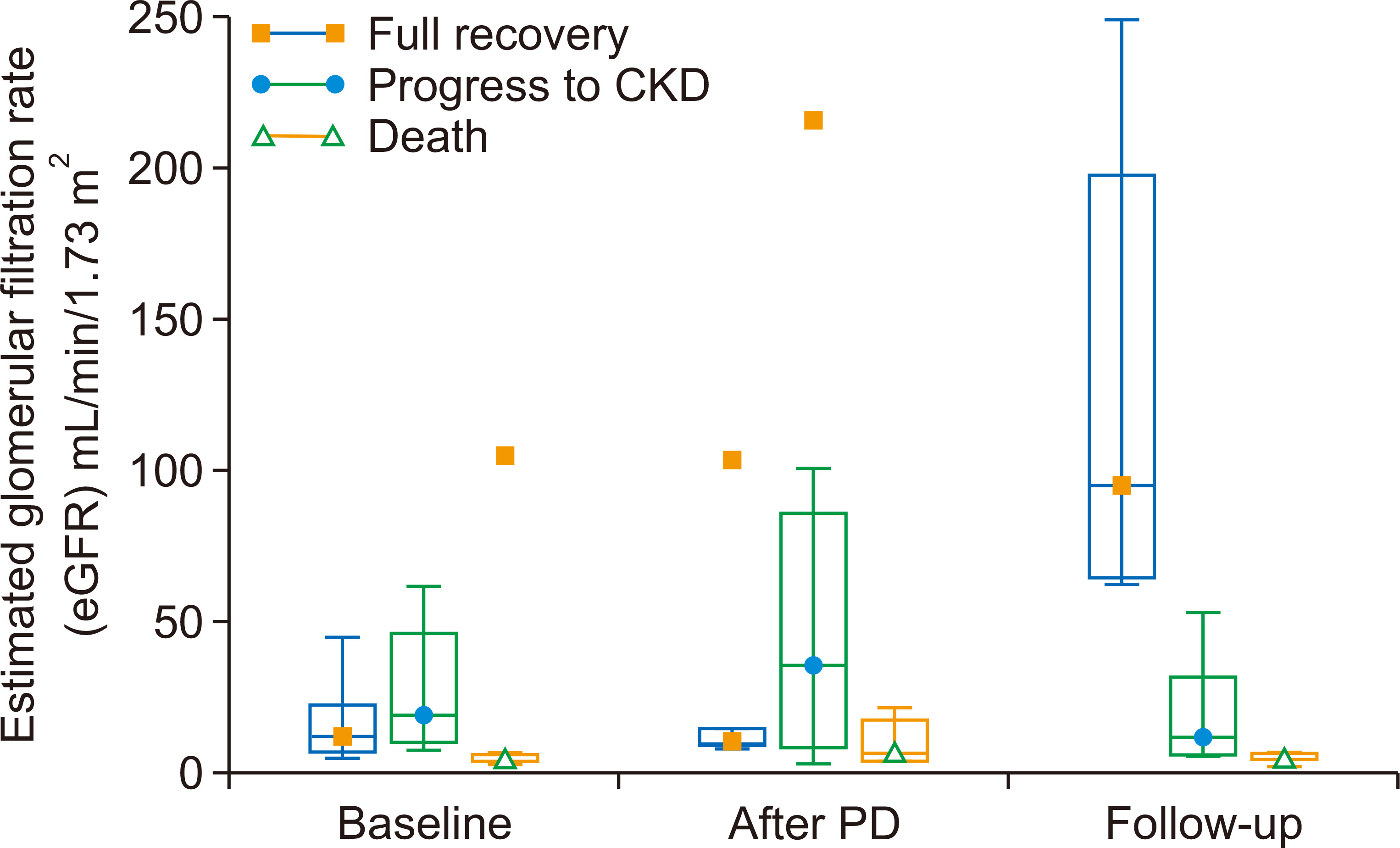
Table 1
Clinical variables of children who were treated with peritoneal dialysis (n = 29)
Table 2
Etiology, severity, and outcomes of AKI among the study children
| Total AKIa | Conservative and renal replacement therapy (n = 102) | |||
|---|---|---|---|---|
|
|
|
|||
| Management | Conservative (n = 73) | Renal replacement therapy (PD) (n = 29) | P value | |
| KDIGO Stage 1 | 46 | |||
| KDIGO Stage 2 | 19 | |||
| KDIGO Stage 3 | 8 | 29 | ||
| Median age (IQR), yr | 7 (3 to 10.5) | 6 (3 to 11) | 0.853 | |
| Range (age) | 3 mo to 17.5 yr | 6 mo to 16 yr | ||
| Etiology | ||||
| Septicemia | 43 | 11 | 0.080 | |
| Post urethral valve | 4 | 3 | 0.402 | |
| Malaria | 5 | 4 | 0.269 | |
| Nephrotic syndrome | 7 | 5 | 0.314 | |
| Diarrheal disease | 6 | 2 | 0.050 | |
| Acute glomerulonephritis | 5 | 3 | 0.685 | |
| Hemolytic uremic syndrome | 3 | 1 | 1.000 | |
| Outcomes (survival/mortality) | 73/0 | 26/3 | 0.021 | |
Table 3
Indications for peritoneal dialysis (n = 29)
| Indication | n (%) |
|---|---|
| Azotemia | 15 (51.7) |
| Uremic encephalopathy | 15 (51.7) |
| Hyperkalemia | 3 (10.3) |
| Anuria | 9 (31.0) |
| Persistent hypertension | 1 (3.4) |
Table 4
Clinical characteristics and causes of mortality among children who underwent acute peritoneal dialysis
| Case | Age and sex | Etiology of AKI | AKI-related complications | Duration of hospitalization (d) | Remark including possible cause of death |
|---|---|---|---|---|---|
| In-hospital mortality | |||||
| Patient one | 1 yr, male | Sepsis | Pulmonary edema, encephalopathy | 1 | MODS |
| Patient two | Six mo, male | Post urethral valve with sepsis | Encephalopathy | 20 | MODS |
| Patient three | 1 yr, male | Sepsis | Pulmonary edema, encephalopathy | 13 | MODS |
| Post-hospitalization mortalitya | |||||
| Patient four | 11 yr, female | Pelviureteric junction obstruction | Uremic syndrome, anuria | 15 | Died six months later from unknown cause of death |
| Patient five | 3 yr, female | Pelvic ureteric with sepsis | Azotemia, anuria | 10 | Died 4 months later from febrile illness |
| Patient six | 2 yr, female | Malaria | Hyperkalemia, azotemia | 12 | Died 22 months later from illness |
| Patient seven | 16 yr, male | Nephrotic syndrome | Anuria, hypertension, azotemia | 11 | Died 36 months later from ESKD |
| Patient eight | 9 yr, male | Nephrotic syndrome | Encephalopathy | 14 | Died 10 months later from unknown cause of death |
Table 5
Multivariate analysis of factors associated with survival
References
2. Cleto-Yamane TL, Gomes CLR, Suassuna JHR, Nogueira PK. 2019;Acute kidney injury epidemiology in pediatrics. Braz J Nephrol 41:275–283.

3. Susantitaphong P, Cruz DN, Cerda J, et al. Acute Kidney Injury Advisory Group of the American Society of Nephrology. 2013;World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 8:1482–1493.



4. Sutherland SM, Ji J, Sheikhi FH, et al. 2013;AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8:1661–1669.



5. Tanyildiz M, Ekim M, Kendirli T, et al. 2017;Acute kidney injury in congenital cardiac surgery: pediatric risk-injury-failure-loss-end-stage renal disease and Acute Kidney Injury Network. Pediatr Int 59:1252–1260.


6. Hoste EAJ, Kellum JA, Selby NM, et al. 2018;Global epidemiology and outcomes of acute kidney injury. Nat Rev Nephrol 14:607–625.


7. Lunyera J, Kilonzo K, Lewington A, Yeates K, Finkelstein FO. 2016;Acute kidney injury in low-resource settings: barriers to diagnosis, awareness, and treatment and strategies to overcome these barriers. Am J Kidney Dis 67:834–840.


8. Macedo E, Cerdá J, Hingorani S, et al. 2018;Recognition and management of acute kidney injury in children: the ISN 0by25 Global Snapshot study. PLoS One 13:e0196586.



9. Mehta RL, Cerdá J, Burdmann EA, et al. 2015;International Society of Nephrology's 0by25 initiative for acute kidney injury (zero preventable deaths by 2025): a human rights case for nephrology. Lancet 385:2616–2643.


10. Olowu WA, Niang A, Osafo C, et al. 2016;Outcomes of acute kidney injury in children and adults in sub-Saharan Africa: a systematic review. Lancet Glob Health 4:e242–e250.


11. Esezobor CI, Ladapo TA, Lesi FE. 2014;Peritoneal dialysis for children with acute kidney injury in Lagos, Nigeria: experience with adaptations. Perit Dial Int 34:534–538.



12. Abdelraheem M, Ali el-T, Osman R, et al. 2014;Outcome of acute kidney injury in Sudanese children - an experience from a sub-Saharan African unit. Perit Dial Int 34:526–533.



13. Mishra OP, Gupta AK, Pooniya V, Prasad R, Tiwary NK, Schaefer F. 2012;Peritoneal dialysis in children with acute kidney injury: a developing country experience. Perit Dial Int 32:431–436.



14. Abraham G, Varughese S, Mathew M, Vijayan M. 2015;A review of acute and chronic peritoneal dialysis in developing countries. Clin Kidney J 8:310–317.



15. Adedoyin OT, Ibrahim OR, Abdurrahman LO, et al. 2015;Peritoneal dialysis in children with acute kidney injury: the Ilorin experience. Afr J Paediatr Nephrol 2:72–76.
16. Anochie IC, Eke FU. 2006;Paediatric acute peritoneal dialysis in southern Nigeria. Postgrad Med J 82:228–230.



17. Kilonzo KG, Ghosh S, Temu SA, et al. 2012;Outcome of acute peritoneal dialysis in northern Tanzania. Perit Dial Int 32:261–266.



18. Schwartz GJ, Work DF. 2009;Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol 4:1832–1843.


19. Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. 2012;KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl 2:1–138.
20. Levey AS, Coresh J, Balk E, et al. National Kidney Foundation. 2003;National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 139:137–147.


21. Long TE, Helgason D, Helgadottir S, et al. 2020 Feb 1 Mild Stage 1 post-operative acute kidney injury: association with chronic kidney disease and long-term survival. Clin Kidney J [Epub]. Doi: 10.1093/ckj/sfz197.

22. Katibi OS, Adedoyin OT, Anoba S, et al. 2013;Current trends in the management of acute kidney injury in children. Niger J Paediatr 40:314–320.
23. Gullipalli P, Anjani A. 2015;Spectrum of paediatric acute kidney injury-a referral hospital experience in a developing nation. IOSR J Dent Med Sci 14:80–87.
24. Duran PA, Concepcion LA. 2014;Survival after acute kidney injury requiring dialysis: long-term follow up. Hemodial Int 18 Suppl 1:S1–S6.

25. Moxey-Mims M. 2018;Kidney disease in African American children: biological and nonbiological disparities. Am J Kidney Dis 72(5 Suppl 1):S17–S21.


26. Devarajan P, Jefferies JL. 2016;Progression of chronic kidney disease after acute kidney injury. Prog Pediatr Cardiol 41:33–40.



27. Fiorentino M, Grandaliano G, Gesualdo L, Castellano G. 2018;Acute kidney injury to chronic kidney disease transition. Contrib Nephrol 193:45–54.


28. Ademola AD, Asinobi AO, Ogunkunle OO, Yusuf BN, Ojo OE. 2012;Peritoneal dialysis in childhood acute kidney injury: experience in southwest Nigeria. Perit Dial Int 32:267–272.



29. Pedersen KR, Hjortdal VE, Christensen S, et al. 2008;Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int Suppl (108):S81–S86.


30. Gunnam S, Gullipalli P. 2018;Therapeutic efficacy of peritoneal dialysis in pediatric acute kidney injury patients in a referral hospital. IOSR J Dent Med Sci 17:41–46.
31. Choi HY, Lee JE, Han SH, et al. 2010;Association of inflammation and protein-energy wasting with endothelial dysfunction in peritoneal dialysis patients. Nephrol Dial Transplant 25:1266–1271.


32. Leinig CE, Moraes T, Ribeiro S, et al. 2011;Predictive value of malnutrition markers for mortality in peritoneal dialysis patients. J Ren Nutr 21:176–183.


33. Al-Othman AM, Al-Naseeb AJM, Almajwal AM, et al. 2016;Association of malnutrition in peritoneal dialysis patients of Saudi Arabia. Arab J Chem 9 Suppl 2:S1059–S1062.





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















