| Kidney Res Clin Pract > Volume 36(3); 2017 > Article |
|
Abstract
Anemia, complicating the course of chronic kidney disease, is a significant parameter, whether interpreted as subjective impairment or an objective prognostic marker. Renal anemia is predominantly due to relative erythropoietin (EPO) deficiency. EPO inhibits apoptosis of erythrocyte precursors. Studies using EPO substitution have shown that increasing hemoglobin (Hb) levels up to 10–11 g/dL is associated with clinical improvement. However, it has not been unequivocally proven that further intensification of erythropoiesis stimulating agent (ESA) therapy actually leads to a comprehensive benefit for the patient, especially as ESAs are potentially associated with increased cerebro-cardiovascular events. Recently, new developments offer interesting options not only via stimulating erythropoeisis but also by employing additional mechanisms. The inhibition of activin, a member of the transforming growth factor superfamily, has the potential to correct anemia by stimulating liberation of mature erythrocyte forms and also to mitigate disturbed mineral and bone metabolism as well. Hypoxia-inducible factor prolyl hydroxylase inhibitors also show pleiotropic effects, which are at the focus of present research and have the potential of reducing mortality. However, conventional ESAs offer an extensive body of safety evidence, against which the newer substances should be measured. Carbamylated EPO is devoid of Hb augmenting effects whilst exerting promising tissue protective properties. Additionally, the role of hepcidin antagonists is discussed. An innovative new hemodialysis blood tube system, reducing blood contact with air, conveys a totally different and innocuous option to improve renal anemia by reducing mechanical hemolysis.
Until the end of the 1980s, correction of renal anemia depended on blood transfusions which carry the inherent risk of transmission of viral, but also non-viral infection. The introduction of erythropoiesis stimulating agents (ESAs) based on recombinant human erythropoietin (EPO) enabled dose dependent correction of anemia via medication [1,2]. First in 1989, epoetin-α and then second, in 1990, epoetin-β were introduced to international markets. Initially, marked euphoria erupted also raising hopes that complete correction of renal anemia would benefit patients in comparison to partial correction [3] as substitution of a pathophysiological absolute or relative EPO deficit was apparently possible [4].
Physiologically, EPO inhibits apoptosis of erythrocyte precursors [5,6]. Furthermore, the condition of relative EPO deficiency can deteriorate in the presence of inflammation with release of cytokines, e.g. interleukin (IL-1 and IL-6) and also tumor necrosis factor (TNF-alpha), as these factors can not only induce premature destruction of erythroid progenitor cells but also a reduction in erythropoietin receptors on the surface of stem cells [7–9].
These ideas, which were driven by perception of pathophysiology, were confirmed by initial clinical studies in the first half of the 1990s which recruited patients with severe anemia. Partial correction of anemia had positive effects, for example regarding cognitive functions [10], a reduction in left ventricular hypertrophy (LVH) [11], an increase in physical capacity including augmented oxygen uptake [12] and reduction in hospital admissions [13]. The CanEPO-Study represented a landmark [14], in which patients were randomized into three groups, including either placebo or erythropoietin targeting a hemoglobin (Hb)-concentration of 9.5–11.0 g/dL (i.e., partial correction) or 11.5–13.0 g/dL (full correction); mean Hb-levels of 7.4 g/dL (n = 40), 10.2 g/dL (n = 40) respectively 11.7 g/dL (n = 38) were achieved. The majority of improvement in anemic symptoms was noted in the group with Hb-values approximating 10.2 g/dL with no significant further improvement at 11.7 g/dL. Eleven of 78 patients treated with erythropoietin had their sites of access clotted compared with only one of 40 patients given placebo. However, the study was principally underpowered to sufficiently evaluate cerebral insults and mortality, and these events were not reported.
Two decades later, Parfrey et al [15] published a meta-analysis confirming that increasing Hb levels from less than 10 g/dL to 10–12 g/dL in chronic kidney disease (CKD) patients induced significantly pronounced regression of LVH (Fig. 1A). Regression of LVH started within the first 6 months of treatment [16]. However, augmenting Hb levels from between 10 and 12 g/dL to higher than 12 g/dL did not induce significant further regression of LVH (Fig. 1B).
Additionally, Parfrey et al [17] in 2005 with 596 randomized hemodialysis patients, CHOIR in 2006 with 1,433 [18] and CREATE with 600 randomized participants with CKD stage 3 and 4 [19] could also not show any convincing advantages attempting to normalize Hb levels (i.e., 13.3 g/dL) vs. partial anemia correction (i.e., 10.9 g/dL). However, a slight increase in cerebral insults [17] and in cardiovascular mortality [18] was detected.
In 2009, results of the largest ESA study were published (TREAT) [20]. TREAT comprised more than 4,000 diabetics with CKD stage 3 and 4 randomly assigning 2,012 patients to darbepoetin alfa to achieve a Hb level of approximately 13 g/dL and 2,026 patients to placebo, with rescue darbepoetin alfa when the Hb level was less than 9.0 g/dL. The study effectively compared a group achieving an Hb-level of approximately 12.5 g/dL with a group achieving approximately 11 g/dL. A cerebrovascular insult occurred in 101 patients with high Hb in the verum group vs. 53 patients on placebo, equating to 5.0% respectively 2.6% (P < 0.001).
Interestingly, a recent evaluation of 24,957 hemodialysis patients treated in the United States (US) Dialysis Clinic Inc. facilities between 2011 and 2014 revealed that although the percentage of patients receiving their ESA subcutaneously had increased from 41% to 69% no difference in dosage was detected between the subcutaneous and intravenous application routes. Furthermore, increased ESA doses were associated with an increase in hospital admissions and mortality, however, there was also no difference between subcutaneous and intravenous routes of administration [21]. Thus, the previously postulated impact of EPO peak serum levels as a pathophysiological detrimental mechanism of cardiovascular damage is somewhat questioned.
The results of these three studies performed on patients with CKD 3 and 4 were comparable to the findings of Besarab et al [22] in the NHCT-study on 1,233 randomized hemodialysis patients showing increased rates of vessel complications and a questionable improvement in quality of life targeting complete anemia correction [23]. As a consequence of the combined results, a long-term debate ensued regarding the impact of ESA-hyporesponse and of comorbities.
The potential of treatment with EPO to reduce progression of CKD has been researched intensely. Indeed, some preclinical data suggested that ESAs might be renoprotective via the EPO-receptor in non-hematopoietic renal tissue resulting in antiapoptotic effects [24–26]. In 2011, the PRIMAVERA study was initiated to examine the renoprotective effect of continuous erythropoiesis receptor activator (CERA) in clinical practice [27]. However, although the concept appears appealing, as yet, a recent meta-analysis of pertinent studies utilizing conventional ESAs has not shown any significant decrease in CKD progression [28].
Information derived from the US Renal Data System (USRDS) [29] and from the German Quality in Nephrology (QIN) data base show that recommendations of reduced Hg targets [30–32] have led to a reduction in the average Hb levels in standard everyday clinical hemodialysis practice. In the US, Hb fell markedly from 12 g/dL in 2007 to circa 10.5 g/dL in 2015. The German QIN group comprises approximately 200 KfH non-profit dialysis centers catering for circa 17,000 patients equating to 1/3 of German dialysis population. Results from QIN show a decrease from ca. 11.6 g/dL in 2007 to approximately 11.2 g/dL in 2015 in hemodialysis patients as compared to a fall from 11.9 g/dL to 11.3 g/dL in peritoneal dialysis patients during the same time period (Fig. 2).
Fig. 3 shows that in South Korea, Hb levels in hemodialysis and peritoneal dialysis patients have increased over the first decade after 2000 when the use of ESAs was prevalent (Korean end-stage renal disease [ESRD] registry data). However, they do demonstrate a plateau at approximately 10.5 g/dL and do not show a further increase during the recent decade. These results are mostly related to the reimbursement system of the Korean government.
An American analysis has shown that uninterrupted prescription of ESAs in CKD stage 3–5 non dialysis during the years 2011 until 2013 has decreased from 9.7% to 3.4% [33]. Accordingly, the percentage of patients not treated with ESAs has increased from 70.6% to 87.3%.
Consistently, a retrospective analysis of Hb-courses and of clinical cerebro-cardiovascular events registered in the US Medicare Program has estimated that the reduction in mean Hb from approximately 11.9 to 10.6 g/dL during the years 2005 to 2012 has led to a slight reduction in cardiovascular events [34]. However, this development has been accompanied by an increase in blood transfusions. Data from the USRDS, which includes information on prevalent dialysis patients covered by Medicare Program, show a 24% increase from 2.4% in September 2010 to 3.0% in September 2011 in the proportion of transfusion claims [35]. Transfusions are especially problematic in kidney transplant candidates due to the potential of inducing antibodies [36]. However, the Dialysis Outcomes and Practice Patterns Study (DOPPS) data extending to 2014 appear to demonstrate a plateau in the transfusion rates (Fig. 4).
As an alternative to darbepoetin alfa and pegylated epoetin beta, polysialic acid (PSA) derived from Escherichia coli has been bonded to epoetin alfa allowing once monthly administration. Several smaller studies have been completed without any indication of unexpected adverse effects [37]. PSA-epoetin alfa is presently being studied clinically in India and Russia.
Another option is being followed by Genexine (Seongnam, Korea), by fusing epoetin to hybrid human crystallisable fragment (Fc) molecules, thus, increasing stability and plasma half-life [38].
EPO mimetic peptides are synthetic polypeptides comprising approximately 20 amino acids showing no resemblance in their sequences to EPO or conventional ESAs. However, they act via the EPO receptor. The first of these products, peginesatide, had passed the clinical trials proving non-inferiority to darbepoetin alfa. However, following licensing and mass distribution in the US, peginesatide was withdrawn after 0.02% of patients died of hypersensitivity reactions on the first intravenous administration [39].
An alternative approach is being examined by genetically linking erythropoietin mimetic peptides (EMP) to an immunoglobulin G (IgG)-based scaffold, resulting in a fusion protein. Two such formulations are CNTO 528 and CNTO 530, both containing two EMP molecules. Thus far, only animal experiments have been reported [40].
Since the turn of the millennium, research has identified additional pathways and mechanisms to modulate and enhance erythropoiesis.
In advanced renal failure, particularly in patients with ESRD, proteins are increasingly carbamylated because of reacting with cyanate. Carbamylation results in transformation of all lysines in the EPO molecule into homocitrulline. Of special interest is that carbamylated EPO (CEPO) can convey tissue protective effects, e.g. neuroprotection against ischemia and amelioration of acute renal failure, whilst being devoid of erythropoietic properties, thus, offering a potential therapeutic option independent of Hb augmentation [41].
Activins are dimers of beta-type chains and belong to the transforming growth factor beta (TGF-β) superfamily. Several members of the TGF-β family, e.g. acitivin, bone morphogenic protein (BMP) amongst other proteins, also contribute to erythropoiesis, sotatercept (ACE-011 developed by the company Acceleron in co-operation with Celgene) is a so-called fusion protein, comprised of an extracellular chain of the activin receptor IIA and the Fc domain of human IgG1. Sotatercept binds circulating activin and related proteins, e.g. BMP 10 and BMP 11, thus, inhibiting activation of the endogenic, membranous receptors (ActRIIA) of activin [42,43]. Activin is a protein complex which stimulates biosynthesis and secretion of follicle stimulating hormone (FSH) and, furthermore, multiple other functions inducing cell proliferation, apoptosis [44], wound healing [45] and bone metabolism. Physiologically, activin is secreted primarily in the embryonal phase with no noteworthy production in healthy later life. However, activin production is reinstated in peritubular myofibroblasts in states of kidney failure, thus, in turn, suppressing for example tubular Klotho-expression [46].
A double blind, randomized escalation study of 48 women showed a reversal of bone loss and a reduction in the extent of osteoporosis [47]. Additionally, an increase in Hb was noticed. The exact mechanism is still being analyzed, however, sotatercept has an effect on cellular and soluble factors in the bone marrow, e.g. the expression of angiotensin II which can stimulate erythropoiesis directly and indirectly via EPO production [48]. Sotatercept induces increased liberation of the mature erythrocyte forms. Additionally, sotatercept reduces the expression of the vascular endothelial growth factor (VEGF), which is regarded as an inhibitor of erythropoiesis and an agonist of carcinogenesis. Furthermore, sotatercept can inhibit hepcidin transcription in the liver [49].
Preliminary studies with sotatercept in dialysis patients have been completed showing a dose dependent increase in Hb and a reduction in extraosseous calcification [50]. Presently, the predialysis phase is at the center of interest. Other research groups have reported reduced cyst growth via activin-inhibition in polycystic kidney degeneration [51]. Recently, studies have started with luspatercept, which represents an ActRIIB fusion protein and is a parallel TNF-β ligand trap binding TGF-β proteins [52]. It also augments erythropoiesis in combination with EPO.
In contrast to ESAs which increase EPO levels via exogenic administration, hypoxia inducible transcription factors (HIF) can stimulate endogenic EPO production [53]. There are 3 isoforms of the α-subunit, i.e. HIF-1α, HIF-2α and HIF-3α, of which HIF-2α is the main subunit involved in upregulation of EPO gene expression. HIF-2α is expressed in the peritubular fibroblasts [54]. HIF-1α is expressed in nearly all cells and plays a critical role in the cell-cycle of hematopoietic stem cells [55], contrasting with HIF-2α which shows a more limited distribution. EPO expression is reduced in normoxia as specific dioxygenases support hydroxylation and, therefore, inactivation of α-subunits of HIF-2 (Fig. 5).
However, the signal cascade is not attenuated in hypoxia resulting in an increase in EPO gene expression and as a consequence also in EPO production [56]. In the absence of renal HIF-2, hepatic HIF-2 becomes the major EPO stimulator [57]. The HIF-prolyl-hydroxylase inhibitors (e.g. GSK1278863 [daprodustat], BAY 85-3934 [molidustat], FG 4592 [roxadustat]) exert their effects via the molecular oxygen sensing mechanisms and influence several systems [58]. EPO production induced by HIF leads to the production of erythroferrone by erythroblasts, which in turn down regulates gene expression of liver hepcidin [59,60]. The most significant differences in the mechanism of action are demonstrated in Fig. 6.
Table 1 lists the potential advantages and disadvantages of HIF inhibitors. Other aspects of HF inhibition in comparison to conventional ESA therapy, e.g. reduced peak EPO concentrations on HIF inhibitors, are not listed, as they have not yet been proven to be of practical relevance.
Remarkably, effectiveness is apparently independent of inflammation state, as measured by hepcidin and C reactive protein, and also independent of the initial ESA dosage [61]. Additionally to increasing Hb, HIF-prolyl-hydroxylase inhibitors suppress hepcidin [62,63], whereas this can also be achieved, at least in part, by conventional ESAs [64]. In principle, it has yet to be shown whether the reduction in hepcidin is actually a direct effect of HIF inhibitors or mediated indirectly by augmentation of Hb levels.
Remarkably, several research groups have reported no significant increase in EPO levels on HIF inhibitors during a 24 week observational period [65,66]. Thus, the pleiotropic mechanisms of this class of substance gain importance. Practical advantages possibly result from oral efficacy of the HIF inhibitors, and from an unspecific protective effect, e.g. against phosphate induced vessel calcification [67]. Significant reductions in cholesterol have been reported [68,69]. Present data suggest a significant improvement in quality of life in patients with CKD [70]. Additionally, protective effects have been described in transient ischemic cerebral insults [71]. Therefore, it appears possible that the pleiotropic mechanisms may—for the first time in anemia treatment—result in an unequivocal reduction in mortality. Large international studies are starting to examine longer term effects on CKD patients [72,73].
However, HIFs also activate, additionally to EPO, more than 300 genes, which can also cause substantial side effects. Furthermore, expression of HIF is not restricted to cells that secrete erythropoietin, moreover, it participates in regulation of several hundred genes in all mammalian cell types within a wide range of adaptive responses to oxygenation status [74], including glycolytic enzymes and VEGF which are relevant in all aspects of neoangiogenesis [75]. In this respect, potential promotion of malignancy must be addressed, especially as transcription of the VEGF gene is regulated by HIF-1α and HIF-2α binding to hypoxia response elements [76,77]. Demonstrating the subtle but significant properties of these substances, several chemical inhibitors of HIFs, specifically inhibiting HIF-1α, are being examined as anti-carcinogenic compounds [78,79].
A further aspect of chronic HIF prolyl hydroxylase inhibitors pertains to the potential of inducing pulmonary hypertension. However, this condition typically develops in patients with genetically inherent HIF-2α mutations [80] and has not yet been described in the CKD population on these new compounds [81].
A focus of research lied in gene technology to substitute EPO deficiency. In 2002, Binley et al [82] achieved sustained extra renal and extra hepatic EPO production by injecting the DNA sequence for EPO into mouse muscles. Also, other working groups have delivered interesting results, e.g. with viral transmission of genetic information into fat cells [83] or stem cells [84]. Others researched the possibility of introducing p-DNA (plasmid-DNA) without a viral vector into muscle cells [85] requiring special pre-cautionary measures regarding the security of transmitted DNA and the controllability of Hb levels. However, as a consequence of guidelines generally recommending lower and narrower Hb targets, these techniques including reimplantation of virus-vector-treated autologous subcutaneous tissue for continuous EPO-production (EPODURE-Biopump, later named Transducer Autologous Regenerative Gene Therapy TARGT, Medgenics) [86] have, at least temporarily, lost their appeal in broader application. Usage in ESA-hyporesponse remains to be defined. The TARGTEPO Treatment for Anemia in Peritoneal Dialysis US Trial was terminated in June 2017 having recruited only one patient [87]. Presently, we are awaiting results from the TARGTEPO Treatment for Anemia in CKD and ESRD study which was completed in April 2017 [88].
Hepcidin regulates the flux of iron into plasma by regulating ferroportin expression on the surface of cells, e.g. on the basolateral membrane of duodenal enterocytes and the surface of hepatocytes and macrophages. Surface expression of ferroportin is essential for iron to enter the systemic circulation. Hepcidin binds to ferroportin, which results in internalization and degradation of the complex, effectively reducing iron release from macrophages and preventing duodenal iron absorption. High hepcidin levels occur when iron stores are replete or there is significant inflammation (Fig. 7) [89]. Hepcidin levels increase progressively with severity of CKD, with predialysis CKD patients having a two- to four-fold elevation, and dialysis patients with a six- to nine-fold increase of hepcidin [90].
As hepcidin plays a key role in regulating iron transport and availability, it has generated considerable interest aiming at developing specific drugs which can readjust and correct the disturbed iron metabolism in states of chronic inflammation, as e.g. in CKD [91,92]. Inhibition of the BMP6, SMAD, HJV and IL-6/STAT cascades is being investigated, albeit at an early stage. Regarding the effects of EPO and HIF inhibitors on hepcidin please refer to the sections above.
Presently, lexaptepid pegol (NOX-H94; anti-hepcidin; Spiegelmer®), which is a pegylated L-stereoisomer RNA aptamer and binds human hepcidin avidly and offers bio-stability as it is resistant to nucleases, appears promising as an hepcidin modulator. In early stage human experiments, lexaptepid has been shown to neutralize human hepcidin, thus, inducing higher serum iron concentrations [93]. Recently, lexaptepid pegol was tested in ESA-hyporesponsive dialysis patients, demonstrating an increase in serum iron and transferrin saturation [94].
Conventional oral iron compounds are generally poorly tolerated and of low efficacy in CKD [95]. Historically, oral supplementation with ferrous salts in the absence of food failed to meet the erythropoietic demands of absolute and functional iron deficiencies in patients with ESRD, largely because of gastrointestinal intolerance that limited dosing to approximately 200 mg elemental iron per day [96]. In this respect, ferric citrate may offer a new option to replenish depleted iron stores [97]. Studies addressing the potential of ferric citrate to primarily bind phosphate in the gut and, thus, lower phosphate burden have employed approximately 5 g ferric citrate per day (5 pills per day) [98]. In these studies, ferric citrate caplets were supplied as 1-g ferric citrate caplets containing 210 mg of ferric iron. Thus, the total elemental iron dosage was approximately 1,050 mg up to 2,520 mg daily, which is significantly higher than the elemental iron dosages utilized in every day clinical practice. Additionally, increased iron absorption has been attributed to an effect of citrate on gut endothelial gut cell tight junctions [99]. Ferric citrate contrasts strongly with sucroferric oxyhydroxide which releases significantly smaller amounts of iron whilst facilitating sustained phosphate binding [100].
This inefficiency of oral iron supplementation has led to the widespread use of intravenous (iv) iron in patients on dialysis, with the Dialysis Outcomes and Practice Patterns Study Practice Monitor reporting approximately 74% of patients on dialysis receiving iv iron in February 2017 [101]. Elemental iron is highly toxic, if released directly into the blood stream. The rate of release of bioactive iron is inversely related to the strengths of the complex, i.e. the stronger the complex, the slower the release of the iron [102]. Thus, great efforts have been undertaken to develop a molecular shell enabling managed release of the elemental iron core, and facilitating administration of higher dosages, e.g. 1 g in one session. Iron gluconate is restricted to 125 mg administered carefully over a longer period of time, e.g. 12.5 mg/minute [103] to avoid anaphylactoid reactions. High weight dextran preparations have been largely abandoned due to the increased propensity to induce potentially life threatening anaphylactic reactions [104]. More recently, several iron intravenous compounds have been developed and are widely available enabling administration of significantly higher doses at comparatively accelerated rates with very low rates of intolerability and hypersensitivity, e.g. ferric carboxymaltose and iron isomaltoside [105–107]. These results are supported, for example, by a study utilizing an ex-vivo model using the whole blood of healthy volunteers and hemodialysis patients in which ferric carboxy-maltose and iron isomaltoside caused less complement activation than iron dextran or iron sucrose [108].
Soluble ferric pyrophosphate (Triferic®) received US Food and Drug Administration approval in 2015 allowing administration via the liquid bicarbonate dialysate replenishing iron losses during dialysis. Ferric pyrophosphate citrate crosses the dialyzer membrane, enters the blood, donates its iron directly to transferrin, and is rapidly cleared from circulation. This provides for optimal iron utilization for erythropoiesis and avoids iron sequestration within reticuloendothelial macrophages [109]. No significant intolerance has been detected in several studies, thus, enabling reduction in additional iron supplementation in dialysis patients [110,111]. Present research is attempting to evaluate the impact and safety of intravenous ferric pyrophosphate infusions administered independently of dialysis [112].
Hemodialysis patients suffer blood loss in the dialysis tubing, partially due to blood- air-contact. A new blood tubing system (Oxyless) reduces blood-air-contact by 99.1%. A study with 142 patients randomized into 2 treatment arms over 14 months, showed a reduction in ESA requirements by 34% (P < 0.01) at month 8 and by 53% (P < 0.05) at month 14 whilst Hb levels remained stable at 11.5 g/dL. Furthermore, iron requirements fell by 12% at month 8 and by 25% (P < 0.05) at month 14 as compared to baseline (Fig. 8) [113].
Regardless of interpretation as a subjective physical restriction or an objective marker, anemia is a significant, prognostic parameter in the development of CKD [114]. ESA studies have shown that augmentation of Hb to 10 to 11 g/dL is associated with clinical advantages. However, it has not been proven that intensified Hb > 11 g/dL utilizing exogenic EPO actually translates into a comprehensive benefit for the patient [115].
New developments promise interesting therapeutic options not only by stimulating erythropoiesis, but also by modulating additional mechanisms. Activin-inhibition offers the potential not only to correct anemia but also to improve mineral and bone disease in CKD (CKD-MBD). HIF prolyl hydroxylase inhibitors have inherent pleiotropic effects which are at the focus of present research. Hepcidin modulation conveys the option to liberate iron metabolism. However, conventional ESAs offer a comprehensive safety profile which should be the future comparison standard for the newer substance classes.
Development of a blood tubing system for hemodialysis represents a completely different method. This improvement in renal anemia is not achieved by stimulating erythropoiesis but by innocent reduction of mechanical hemolysis during dialysis.
We may be standing on the verge of total re-evaluation of renal anemia therapy.
Figure 1
Effects of anemia correction on left ventricular hypertrophy
(A) Significant regression of left ventricular hypertrophy with initial hemoglobin (Hb)-levels of < 10 g/dL and aiming for 10 to 12 g/dL. (B) Non-significant regression of left ventricular hypertrophy with initial Hb levels of 10 to 12 g/dL and aiming for > 12 g/dL. CI, confidence interval.
LVMi, left ventricular mass index.
Adated from the article of Parfrey et al (Clin J Am Soc Nephrol 4:755–762, 2009) [15] with original copyright holder’s permission.
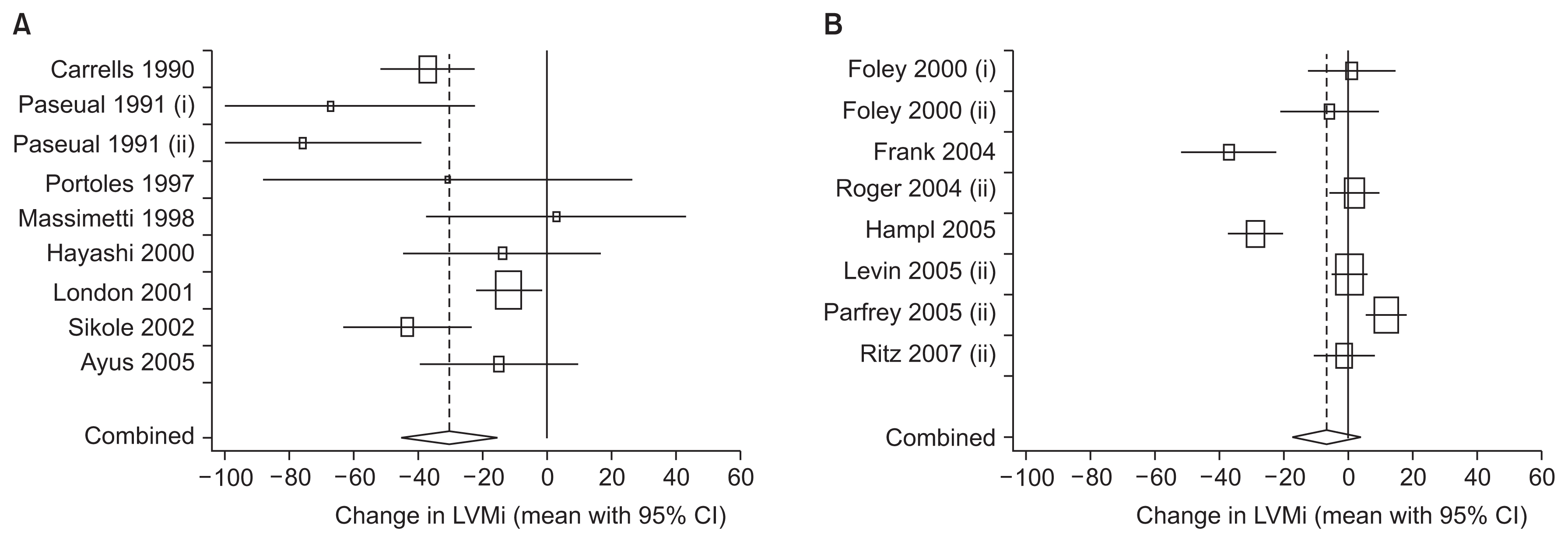
Figure 2
Hemoglobin (Hb) levels in g/dL from 2007 until 2015 (n = approximately 15,000) in KfH-Institution hemodialysis (HD) and peritoneal dialysis (PD) patients in Germany (QIN Data).
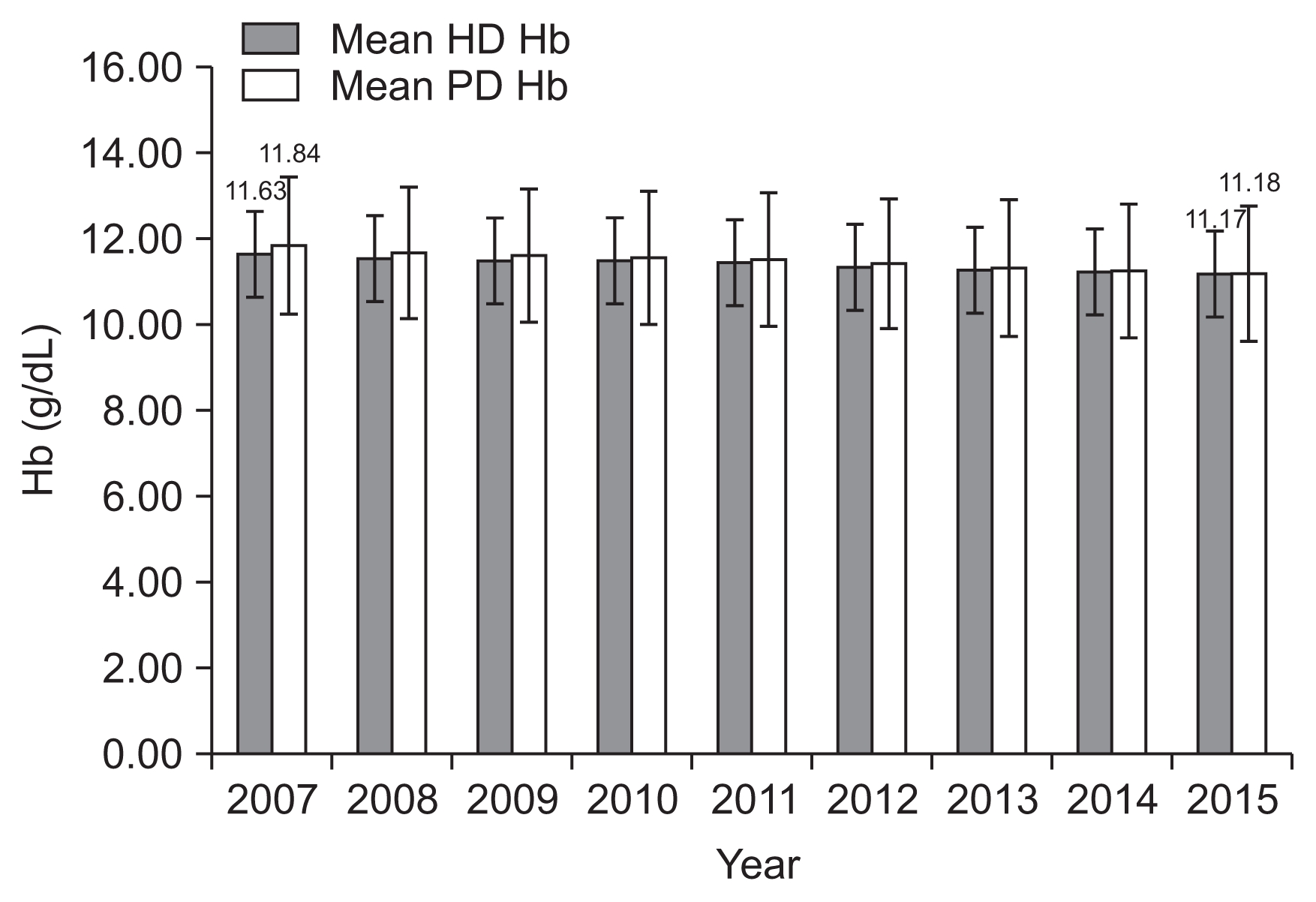
Figure 3
Hemoglobin (Hb) course from 2000 until 2015 in hemodialysis (HD) and peritoneal dialysis (PD) patients in South Korea according to Korean end-stage renal disease registry data.

Figure 4
Dialysis Outcomes and Practice Patterns Study Program data showing a plateau in Medicare red cell blood transfusion claims among facilities with at least 20 patients since 2012 following an increase in previous years (http:/www.dopps.org/DPM).
ESRD, end-stage renal disease.

Figure 5
In the presence of oxygen, prolin hydroxylase oxidates hypoxia inducible transcription factors (HIF)-1α, which is then degraded (ubiquination) via the Hippel-Lindau-protein, thus inhibiting conglomeration of the HIF-1α/HIF-1β-complex, which would otherwise up-regulate the hypoxia-response genes
Adapted from the article of Prchal (N Engl J Med 348:1282–1283, 2003) [53] with original copyright holder’s permission.

Figure 6
Overview of pharmacological sites of actions in erythropoiesis
In contrast to EPO, which influences the survival of early erythrocyte cells, sotatercept induces the liberation of mature erythrocytes.
HIF inhibitors have more mechanisms of action, e.g., augmentation of residual EPO production and reduction of hepcidin. BFU-E, burstforming unit-erythroid; CFU-E, colony-forming unit-erythroid; EPO, erythropoietin; HIF, hypoxia inducible transcription factor; HSC, hematopoietic stem cell; rhEPO, recombinant human EPO. Adapted from the article of Jelkmann (Nephrol Dial Transplant 30:553–559, 2015) [43] with original copyright holder’s permission.

Figure 7
Impact of hepcidin levels on duodenal iron absorption. (A) Normal duodenal iron absorption under conditions of low hepcidin blood levels. (B) Decreased duodenal iron absorption under conditions of high hepcidin levels. Adapted from the article of Larson and Coyne (Kidney Res Clin Pract 32:11–15, 2013) [89] with original copyright holder’s permission.

Figure 8
Reduction in ESA- and iron requirement employing specially constructed dialysis tubing to minimize blood-air-contact
Reverting back to standard tubing resulted in an increase in ESA and iron requirements.
ESA, erythropoiesis stimulating agent; Hb, hemoglobin; IV, intravenous. Adapted from the abstract of Macdougall et al in American Society of Nephrology (ASN) Kidney Week Abstract Supplement (2016, Chicago) with original copyright holder’s permission.

Table 1
Potential advantages and disadvantages of hypoxia inducible transcription factors inhibitors
| Potential benefits | Potential harm |
|---|---|
| Improved iron utilization* | Increase in pulmonary artery pressure* |
| Blood pressure reducation | Destabilization of atherosclerotic plaques and of vascular aneurysms |
| Lipid lowering effect* | Increased vascular calcification |
| Reduced progression of kidney disease | Increased growth of renal cysts |
| Ischemia protection | Tumor progression |
| Improved neo-vascularization |
References
1. Ibrahim HN, Ishani A, Foley RN, Guo H, Liu J, Collins AJ. Temporal trends in red blood transfusion among US dialysis patients, 1992–2005. Am J Kidney Dis 52:1115–1121. 2008;


2. Lawler EV, Bradbury BD, Fonda JR, Gaziano JM, Gagnon DR. Transfusion burden among patients with chronic kidney disease and anemia. Clin J Am Soc Nephrol 5:667–672. 2010;



3. Fishbane S. Erythropoiesis-stimulating agent treatment with full anemia correction: a new perspective. Kidney Int 75:358–365. 2009;


4. Katzung BG. Basic & clinical pharmacology. 10th edition. New York: McGraw Hill; p. 536–538. 2007.
6. Socolovsky M, Fallon AE, Wang S, Brugnara C, Lodish HF. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- mice: a direct role for Stat5 in Bcl-X(L) induction. Cell 98:181–191. 1999;


7. Wang CQ, Udupa KB, Lipschitz DA. Interferon-gamma exerts its negative regulatory effect primarily on the earliest stages of murine erythroid progenitor cell development. J Cell Physiol 162:134–138. 1995;


8. Taniguchi S, Dai CH, Price JO, Krantz SB. Interferon gamma downregulates stem cell factor and erythropoietin receptors but not insulin-like growth factor-I receptors in human erythroid colony-forming cells. Blood 90:2244–2252. 1997;


9. Means RT Jr. Recent developments in the anemia of chronic disease. Curr Hematol Rep 2:116–121. 2003;

11. London GM, Zins B, Pannier B, Naret C, Berthelot JM, Jacquot C, Safar M, Drueke TB. Vascular changes in hemodialysis patients in response to recombinant human erythropoietin. Kidney Int 36:878–882. 1989;


12. Macdougall IC, Lewis NP, Saunders MJ, Cochlin DL, Davies ME, Hutton RD, Fox KA, Coles GA, Williams JD. Long-term cardiorespiratory effects of amelioration of renal anaemia by erythropoietin. Lancet 335:489–493. 1990;


13. Collins AJ, Li S, St Peter W, Ebben J, Roberts T, Ma JZ, Manning W. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39%. J Am Soc Nephrol 12:2465–2473. 2001;


14. Canadian Erythropoietin Study Group. Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. BMJ 300:573–578. 1990;



15. Parfrey PS, Lauve M, Latremouille-Viau D, Lefebvre P. Erythropoietin therapy and left ventricular mass index in CKD and ESRD patients: a meta-analysis. Clin J Am Soc Nephrol 4:755–762. 2009;



16. Ayus JC, Go AS, Valderrabano F, Verde E, de Vinuesa SG, Achinger SG, Lorenzo V, Arieff AI, Luño J. Spanish Group for the Study of the Anemia and Left Ventricular Hypertrophy in Pre-dialysis Patients. Effects of erythropoietin on left ventricular hypertrophy in adults with severe chronic renal failure and hemoglobin <10 g/dL. Kidney Int 68:788–795. 2005;


17. Parfrey PS, Foley RN, Wittreich BH, Sullivan DJ, Zagari MJ, Frei D. Double-blind comparison of full and partial anemia correction in incident hemodialysis patients without symptomatic heart disease. J Am Soc Nephrol 16:2180–2189. 2005;


18. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D. CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355:2085–2098. 2006;


19. Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A. CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355:2071–2084. 2006;


20. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R. TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361:2019–2032. 2009;


21. Gul A, Schrader R, Miskulin D, Paine S, Harford A, Zager P. SA-OR108: Dose requirements, hospitalization, and mortality are similar with subcutaneous versus intravenous erythropoietin administration. In: American Society of Nephrology (ASN) Kidney Week Abstract Supplement; 2016; Chicago. Washington: ASN; 2016;[Abstract].
22. Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339:584–590. 1998;


23. Coyne DW. The health-related quality of life was not improved by targeting higher hemoglobin in the Normal Hematocrit Trial. Kidney Int 82:235–241. 2012;



24. Elliott S, Sinclair AM. The effect of erythropoietin on normal and neoplastic cells. Biologics 6:163–189. 2012;


25. Bahlmann FH, Fliser D. Erythropoietin and renoprotection. Curr Opin Nephrol Hypertens 18:15–20. 2009;


26. Hirata M, Tashiro Y, Aizawa K, Kawasaki R, Shimonaka Y, Endo K. Epoetin beta pegol alleviates oxidative stress and exacerbation of renal damage from iron deposition, thereby delaying CKD progression in progressive glomerulonephritis rats. Physiol Rep 3:e126372015;



27. Fliser D, Dellanna F, Koch M, Seufert J, Witzke O, Hauser IA. The Primavera study protocol design: evaluating the effect of continuous erythropoiesis receptor activator (C.E.R.A.) on renal function in non-anemic patients with chronic kidney disease. Contemp Clin Trials 32:786–792. 2011;


28. Elliott S, Tomita D, Endre Z. Erythropoiesis stimulating agents and reno-protection: a meta-analysis. BMC Nephrol 18:142017;



29. USRDS Annual Data Report 2016 Available at: https://www.usrds.org/adr.aspx. Date accessed: 13 August 2017.
30. FDA Drug Safety Communication. Modified Dosing Recommendations to Improve the Safe Use of Erythropoiesis-Stimulating Agents in Chronic Kidney Disease 2011 Available at: http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Date accessed: 15 November 2011.
31. German Common Federal Committee Announcements. Available at: http://www.g-ba.de/downloads/39-261-1341/2011-06-23_AM-RL-IV_Erythropoese_BAnz.pdf?. Date accessed: 13 August 2017.
32. Kidney Disease: Improving Global Outcomes (KDIGO). KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2:279–335. 2012.
33. Peter WL, Guo H, Kabadi S, Zhao S, Gilbertson DT, Sargent-Heuer LJ, Peng Y, Pendergraft T, Li S. FR-O767: Anemia treatment pattern changes in non-dialysis-dependent chronic kidney disease patients before and after revised Food and Drug Administration label and New Anemia Guidelines for Erythropoiesis-Stimulating Agents. In: American Society of Nephrology (ASN) Kidney Week Abstract Supplement; 2016; Chicago. Washington: ASN; 2016;[Abstract].
34. Chertow GM, Liu J, Monda KL, Gilbertson DT, Brookhart MA, Beaubrun AC, Winkelmayer WC, Pollock A, Herzog CA, Ashfaq A, Sturmer T, Rothman KJ, Bradbury BD, Collins AJ. Epoetin alfa and outcomes in dialysis amid regulatory and payment reform. J Am Soc Nephrol 27:3129–3138. 2016;



35. Cappell KA, Shreay S, Cao Z, Varker HV, Paoli CJ, Gitlin M. Red blood cell (RBC) transfusion rates among US chronic dialysis patients during changes to Medicare end-stage renal disease (ESRD) reimbursement systems and erythropoiesis stimulating agent (ESA) labels. BMC Nephrol 15:1162014;



36. Obrador GT, Macdougall IC. Effect of red cell transfusions on future kidney transplantation. Clin J Am Soc Nephrol 8:852–860. 2013;


37. Smirnov IV, Vorobiev II, Belogurov AA, Genkin DD, Deyev SM, Gabibov AG. Chemical polysialylation of recombinant human proteins. Methods Mol Biol 1321:389–404. 2015;


38. Study to evaluate the efficacy and safety of GX-E2 in the anemic patient diagnosed with chronic kidney disease (CKD) 2014 Available at: https://clinicaltrials.gov/ct2/show/NCT02044653. Date accessed: 13 August 2017.
39. Bennett CL, Jacob S, Hymes J, Usvyat LA, Maddux FW. Anaphylaxis and hypotension after administration of peginesatide. N Engl J Med 370:2055–2056. 2014;



40. Scully MS, Ort TA, James IE, Bugelski PJ, Makropoulos DA, Deutsch HA, Pieterman EJ, van den Hoek AM, Havekes LM, Dubell WH, Wertheimer JD, Picha KM. A novel EPO receptor agonist improves glucose tolerance via glucose uptake in skeletal muscle in a mouse model of diabetes. Exp Diabetes Res 2011:9101592011;



41. Tögel FE, Ahlstrom JD, Yang Y, Hu Z, Zhang P, Westenfelder C. Carbamylated erythropoietin outperforms erythropoietin in the treatment of AKI-on-CKD and other AKI models. J Am Soc Nephrol 27:3394–3404. 2016;



42. Raje N, Vallet SL. Sotatercept, a soluble activin receptor type 2A IgG-Fc fusion protein for the treatment of anemia and bone loss. Curr Opin Mol Ther 12:586–597. 2010;

43. Jelkmann W. The ESA scenario gets complex: from bio-similar epoetins to activin traps. Nephrol Dial Transplant 30:553–559. 2015;


44. Chen YG, Wang Q, Lin SL, Chang CD, Chuang J, Ying SY. Activin signaling and its role in regulation of cell proliferation, apoptosis, and carcinogenesis. Exp Biol Med (May-wood) 231:534–544. 2006;

45. Sulyok S, Wankell M, Alzheimer C, Werner S. Activin: an important regulator of wound repair, fibrosis, and neuro-protection. Mol Cell Endocrinol 225:127–132. 2004;


46. Hruska KA, Seifert M, Sugatani T. Pathophysiology of the chronic kidney disease-mineral bone disorder. Curr Opin Nephrol Hypertens 24:303–309. 2015;



47. Ruckle J, Jacobs M, Kramer W, Pearsall AE, Kumar R, Underwood KW, Seehra J, Yang Y, Condon CH, Sherman ML. Single-dose, randomized, double-blind, placebo-controlled study of ACE-011 (ActRIIA-IgG1) in postmenopausal women. J Bone Miner Res 24:744–752. 2009;


48. Iancu-Rubin C, Mosoyan G, Wang J, Kraus T, Sung V, Hoffman R. Stromal cell-mediated inhibition of erythropoiesis can be attenuated by Sotatercept (ACE-011), an activin receptor type II ligand trap. Exp Hematol 41:155–166.e17. 2013;


49. Finberg KE, Whittlesey RL, Fleming MD, Andrews NC. Down-regulation of Bmp/Smad signaling by Tmprss6 is required for maintenance of systemic iron homeostasis. Blood 115:3817–3826. 2010;



50. A phase 2 study of intravenous or subcutaneous dosing of Sotatercept (ACE-011) in patients with end-stage kidney disease on hemodialysis 2013 Available at: https://www.clinicaltrials.gov/ct2/show/NCT01999582. Date accessed: 13 August 2017.
51. Leonhard WN, Kunnen SJ, Plugge AJ, Pasternack A, Jianu SB, Veraar K, El Bouazzaoui F, Hoogaars WM, Ten Dijke P, Breuning MH, De Heer E, Ritvos O, Peters DJ. Inhibition of activin signaling slows progression of polycystic kidney disease. J Am Soc Nephrol 27:3589–3599. 2016;



52. Mies A, Hermine O, Platzbecker U. Activin receptor II ligand traps and their therapeutic potential in myelodysplastic syndromes with ring sideroblasts. Curr Hematol Malig Rep 11:416–424. 2016;


54. Tanaka T, Nangaku M. Recent advances and clinical application of erythropoietin and erythropoiesis-stimulating agents. Exp Cell Res 318:1068–1073. 2012;


55. Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, Suda T. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7:391–402. 2010;


56. Holdstock L, Meadowcroft AM, Maier R, Johnson BM, Jones D, Rastogi A, Zeig S, Lepore JJ, Cobitz AR. Four-Week studies of oral hypoxia-inducible factor-prolyl hydroxylase inhibitor GSK1278863 for treatment of anemia. J Am Soc Nephrol 27:1234–1244. 2016;


57. Kapitsinou PP, Liu Q, Unger TL, Rha J, Davidoff O, Keith B, Epstein JA, Moores SL, Erickson-Miller CL, Haase VH. Hepatic HIF-2 regulates erythropoietic responses to hypoxia in renal anemia. Blood 116:3039–3048. 2010;



58. Maxwell PH, Eckardt KU. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol 12:157–168. 2016;


59. Liu Q, Davidoff O, Niss K, Haase VH. Hypoxia-inducible factor regulates hepcidin via erythropoietin-induced erythropoiesis. J Clin Invest 122:4635–4644. 2012;



60. Aschemeyer S, Gabayan V, Ganz T, Nemeth E, Kautz L. Erythroferrone and matriptase-2 independently regulate hepcidin expression. Am J Hematol 92:E61–E63. 2017;



61. Haase VH, Spinowitz BS, Pergola PE, Khawaja Z, Chan J, Zuraw Q, Maroni BJ, Farzaneh-Far R. TH-O907: Efficacy and dose requirements of vadadustat are independent of systemic inflammation and prior erythropoiesis-stimulating agent (ESA) dose in patients with non-dialysis dependent chronic kidney disease (NDD-CKD). In: American Society of Nephrology (ASN) Kidney Week Abstract Supplement; 2016; Chicago. Washington: ASN; 2016;[Poster abstract].
62. Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, Poole L, Saikali KG, Saha G, Hemmerich S, Szczech L, Yu KH, Neff TB. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis 67:912–924. 2016;


63. Soni H. Prolyl hydroxylase domain-2 (PHD2) inhibition may be a better therapeutic strategy in renal anemia. Med Hypotheses 82:547–550. 2014;


64. Ashby DR, Gale DP, Busbridge M, Murphy KG, Duncan ND, Cairns TD, Taube DH, Bloom SR, Tam FW, Chapman R, Maxwell PH, Choi P. Erythropoietin administration in humans causes a marked and prolonged reduction in circulating hepcidin. Haematologica 95:505–508. 2010;


65. Holdstock L, Cizman B, Meadowcroft AM, Biswas N, Jones D, Johnson BM, Lepore JJ, Kim SG, Cobitz AR. TH-O908: Daprodustat, a HIF-prolyl-hydroxylase inhibitor, increases and maintains hemoglobin over 24 weeks in anemic chronic kidney disease subjects. In: American Society of Nephrology (ASN) Kidney Week Abstract Supplement; 2016; Chicago. Washington: ASN; 2016;[Abstract].
66. Cobitz AR, Meadowcroft AM, Cizman B, Holdstock L, Biswas N, Jones D, Johnson BM, Lepore JJ, Nossuli AKK. SA-OR114: Daprodustat, a HIF-prolyl-hydroxylase inhibitor, maintains hemoglobin levels over 24 weeks in anemic hemodialysis subjects switching from recombinant human erythropoietin. In: American Society of Nephrology (ASN) Kidney Week Abstract Supplement; 2016; Chicago. Washington: ASN; 2016;[Abstract].
67. Mokas S, Larivière R, Lamalice L, Gobeil S, Cornfield DN, Agharazii M, Richard DE. Hypoxia-inducible factor-1 plays a role in phosphate-induced vascular smooth muscle cell calcification. Kidney Int 90:598–609. 2016;


68. Provenzano R, Besarab A, Wright S, Dua S, Zeig S, Nguyen P, Poole L, Saikali KG, Saha G, Hemmerich S, Szczech L, Yu KH, Neff TB. Roxadustat (FG-4592) versus epoetin alfa for anemia in patients receiving maintenance hemodialysis: a phase 2, randomized, 6- to 19-week, open-Label, active-comparator, dose-ranging, safety and exploratory efficacy study. Am J Kidney Dis 67:912–924. 2016;


69. Gupta N, Wish JB. Hypoxia-Inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis 69:815–826. 2017;


70. Szczech L, Hemmerich S, Besarab A, Saikali KG, Poole L, Peony Yu KH, Neff TB. TH-OR039: Anemia correction with roxadustat improves Health Related Quality of Life (HRQOL) in chronic kidney disease (CKD) patients. In: American Society of Nephrology (ASN) Kidney Week Abstract Supplement; 2015; San Diego. Washington: ASN; 2015;[Abstract].
71. Chen RL, Ogunshola OO, Yeoh KK, Jani A, Papadakis M, Nagel S, Schofield CJ, Buchan AM. HIF prolyl hydroxylase inhibition prior to transient focal cerebral ischaemia is neuroprotective in mice. J Neurochem 131:177–189. 2014;


72. Anemia Studies in Chronic Kidney Disease: Erythropoiesis Via a Novel Prolyl Hydroxylase Inhibitor Daprodustat-Dialysis (ASCEND-D) 2016 Available at: https://clini-caltrials.gov/ct2/show/NCT02879305. Date accessed: 13 August 2017.
73. Locatelli F, Fishbane S, Block GA, Macdougall IC. Targeting hypoxia-inducible factors for the treatment of anemia in chronic kidney disease patients. Am J Nephrol 45:187–199. 2017;


74. Schödel J, Oikonomopoulos S, Ragoussis J, Pugh CW, Ratcliffe PJ, Mole DR. High-resolution genome-wide mapping of HIF-binding sites by ChIP-seq. Blood 117:e207–e217. 2011;



75. Bonomini M, Del Vecchio L, Sirolli V, Locatelli F. New treatment approaches for the anemia of CKD. Am J Kidney Dis 67:133–142. 2016;


76. Ratcliffe PJ. Oxygen sensing and hypoxia signalling pathways in animals: the implications of physiology for cancer. J Physiol 591:2027–2042. 2013;



77. Krock BL, Skuli N, Simon MC. Hypoxia-induced angio-genesis: good and evil. Genes Cancer 2:1117–1133. 2011;



78. Lee K, Kim HM. A novel approach to cancer therapy using PX-478 as a HIF-1α inhibitor. Arch Pharm Res 34:1583–1585. 2011;


79. Hu Y, Liu J, Huang H. Recent agents targeting HIF-1α for cancer therapy. J Cell Biochem 114:498–509. 2013;


80. Bond J, Gale DP, Connor T, Adams S, de Boer J, Gascoyne DM, Williams O, Maxwell PH, Ancliff PJ. Dysregulation of the HIF pathway due to VHL mutation causing severe erythrocytosis and pulmonary arterial hypertension. Blood 117:3699–3701. 2011;


81. Maxwell PH, Eckardt KU. HIF prolyl hydroxylase inhibitors for the treatment of renal anaemia and beyond. Nat Rev Nephrol 12:157–168. 2016;


82. Binley K, Askham Z, Iqball S, Spearman H, Martin L, de Alwis M, Thrasher AJ, Ali RR, Maxwell PH, Kingsman S, Naylor S. Long-term reversal of chronic anemia using a hypoxia-regulated erythropoietin gene therapy. Blood 100:2406–2413. 2002;


83. Mizukami H, Mimuro J, Ogura T, Okada T, Urabe M, Kume A, Sakata Y, Ozawa K. Adipose tissue as a novel target for in vivo gene transfer by adeno-associated viral vectors. Hum Gene Ther 17:921–928. 2006;


84. Eliopoulos N, Gagnon RF, Francois M, Galipeau J. Erythropoietin delivery by genetically engineered bone marrow stromal cells for correction of anemia in mice with chronic renal failure. J Am Soc Nephrol 17:1576–1584. 2006;


85. Sebestyén MG, Hegge JO, Noble MA, Lewis DL, Herweijer H, Wolff JA. Progress toward a nonviral gene therapy protocol for the treatment of anemia. Hum Gene Ther 18:269–285. 2007;



86. Besarab A, Nissenson AR, Schwartz D, Pearlman AL, Ng P, Elhalel M. F-FC 270: Erythropoiesis sustained 12 months by the EPODURE biopump in patients with chronic kidney disease: further results of phase I/II proof of concept trial. In: American Society of Nephrology (ASN) Renal Week Abstract Supplement; 2010; Denver. Washington: ASN; 2010;[Abstract].
87. TARGTEPO treatment for anemia in PD US Trial 2015 Available at : https://clinicaltrials.gov/ct2/show/NCT02468414. Date accessed: 13 August 2017.
88. TARGTEPO Treatment for Anemia in CKD and ESRD study 2014 Available at: https://clinicaltrials.gov/ct2/show/NCT02117427. Date accessed: 13 August 2017.
89. Larson DS, Coyne DW. Understanding and exploiting hepcidin as an indicator of anemia due to chronic kidney disease. Kidney Res Clin Pract 32:11–15. 2013;



91. Sasu BJ, Cooke KS, Arvedson TL, Plewa C, Ellison AR, Sheng J, Winters A, Juan T, Li H, Begley CG, Molineux G. Antihepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood 115:3616–3624. 2010;


92. Gardenghi S, Renaud TM, Meloni A, Casu C, Crielaard BJ, Bystrom LM, Greenberg-Kushnir N, Sasu BJ, Cooke KS, Rivella S. Distinct roles for hepcidin and interleukin-6 in the recovery from anemia in mice injected with heat-killed Brucella abortus. Blood 123:1137–1145. 2014;



93. van Eijk LT, John AS, Schwoebel F, Summo L, Vauléon S, Zöllner S, Laarakkers CM, Kox M, van der Hoeven JG, Swinkels DW, Riecke K, Pickkers P. Effect of the antihepcidin Spiegelmer lexaptepid on inflammation-induced decrease in serum iron in humans. Blood 124:2643–2646. 2014;



94. Macdougall IC, Rumjon A, Cinco J, Goldstein L, Summo L, Vauléon S, Riecke K. FP 660: Pharmacokinetics and pharmacodynamics of lexaptepid, a novel anti-hepcidin molecule, in ESA-resistanct haemodialysis patients. In: 52nd ERA-EDTA Congress; 2015; London. London: ERA-EDTA; 2015;[Abtract].
95. Hodson EM, Craig JC. Oral iron for patients receiving dialysis: what is the evidence? Semin Dial 27:8–10. 2014;


96. Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, Whittier FC, Linfert DR, Galphin CM, Athreya BP, Nossuli AK, Chang IJ, Blumenthal SS, Manley J, Zeig S, Kant KS, Olivero JJ, Greene T, Dwyer JP. Collaborative Study Group. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol 26:493–503. 2015;


97. Fishbane S, Shah HH. Ferric pyrophosphate citrate as an iron replacement agent for patients receiving hemodialysis. Hemodial Int 21:Suppl 1. S104–S109. 2017;


98. Block GA, Fishbane S, Rodriguez M, Smits G, Shemesh S, Pergola PE, Wolf M, Chertow GM. A 12-week, double-blind, placebo-controlled trial of ferric citrate for the treatment of iron deficiency anemia and reduction of serum phosphate in patients with CKD Stages 3–5. Am J Kidney Dis 65:728–736. 2015;


99. Gupta A. Ferric citrate hydrate as a phosphate binder and risk of aluminum toxicity. Pharmaceuticals (Basel) 7:990–998. 2014;



100. Floege J, Covic AC, Ketteler M, Mann JF, Rastogi A, Spinowitz B, Chong EM, Gaillard S, Lisk LJ, Sprague SM. Sucroferric Oxyhydroxide Study Group. Long-term effects of the iron-based phosphate binder, sucroferric oxyhydroxide, in dialysis patients. Nephrol Dial Transplant 30:1037–1046. 2015;



101. IV iron use in USA 2017 Available at: http://www.dopps.org/DPM/Default.aspx. Date accessed: 13 August 2017.
102. Auerbach M, Coyne D, Ballard H. Intravenous iron: from anathema to standard of care. Am J Hematol 83:580–588. 2008;


103. Michael B, Coyne DW, Fishbane S, Folkert V, Lynn R, Nissenson AR, Agarwal R, Eschbach JW, Fadem SZ, Trout JR, Strobos J, Warnock DG. Ferrlecit Publication Committee: Sodium ferric gluconate complex in hemodialysis patients: adverse reactions compared to placebo and iron dextran. Kidney Int 61:1830–1839. 2002;


104. Walters BA, Van Wyck DB. Benchmarking iron dextran sensitivity: reactions requiring resuscitative medication in incident and prevalent patients. Nephrol Dial Transplant 20:1438–1442. 2005;


105. Avni T, Bieber A, Grossman A, Green H, Leibovici L, Gafter-Gvili A. The safety of intravenous iron preparations: systematic review and meta-analysis. Mayo Clin Proc 90:12–23. 2015;

106. Roger SD, Gaillard CA, Bock AH, Carrera F, Eckardt KU, Van Wyck DB, Cronin M, Meier Y, Larroque S, Macdougall IC. Safety of intravenous ferric carboxymaltose versus oral iron in patients with nondialysis-dependent CKD: an analysis of the 1-year FIND-CKD trial. Nephrol Dial Transplant 2017 Feb 27 [Epub ahead of print].
107. Biggar P, Leistikow F, Walper A. A prospective observational study of effectiveness and safety of iron isomaltoside in patients with chronic renal failure and iron deficiency anemia. Clin Nephrol 86:310–318. 2016;


108. Hempel JC, Poppelaars F, Gaya da Costa M, Franssen CF, de Vlaam TP, Daha MR, Berger SP, Seelen MA, Gaillard CA. Distinct in vitro complement activation by various intravenous iron preparations. Am J Nephrol 45:49–59. 2017;


109. Gupta A, Amin NB, Besarab A, Vogel SE, Divine GW, Yee J, Anandan JV. Dialysate iron therapy: infusion of soluble ferric pyrophosphate via the dialysate during hemodialysis. Kidney Int 55:1891–1898. 1999;


110. Gupta A, Lin V, Guss C, Pratt R, Ikizler TA, Besarab A. Ferric pyrophosphate citrate administered via dialysate reduces erythropoiesis-stimulating agent use and maintains hemoglobin in hemodialysis patients. Kidney Int 88:1187–1194. 2015;



111. Fishbane SN, Singh AK, Cournoyer SH, Jindal KK, Fanti P, Guss CD, Lin VH, Pratt RD, Gupta A. Ferric pyrophosphate citrate (Triferic™) administration via the dialysate maintains hemoglobin and iron balance in chronic hemodialysis patients. Nephrol Dial Transplant 30:2019–2026. 2015;



112. Pratt RD, Swinkels DW, Ikizler TA, Gupta A. Pharmacokinetics of ferric pyrophosphate citrate, a novel iron salt, administered intravenously to healthy volunteers. J Clin Pharmacol 57:312–320. 2017;


113. Macdougall IC, Sousa A, Ryzlewicz T, Becker FF, Fairburn-Beech A, Kilgallon W. SA-O999: Improving anemia therapy in hemodialysis patients. In: American Society of Nephrology (ASN) Kidney Week Abstract Supplement; 2016; Chicago. Washington: ASN; 2016;[Abstract].



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















