| Kidney Res Clin Pract > Volume 31(4); 2012 > Article |
|
Abstract
Background
Herein, the significance of post-transplant glomerulonephritis (PTGN) has been revisited to investigate whether PTGN induces allograft failure. The aim of this study was to identify the incidence of PTGN and its association with allograft failure, as well as to analyze the risk factors for PTGN.
Methods
Among the 996 Korean patients who underwent kidney transplantation in a multicenter cohort from 1995 to 2010, 764 patients were enrolled in this study.
Results
The incidence rate of PTGN was 9.7% and 17.0% at 5 and 10 years of follow-up, respectively. PTGN was diagnosed in 17.8% of the recipients with results of biopsy tests or clinical diagnosis identifying glomerular diseases as the underlying cause, compared with 0.0%, 4.4%, 4.9%, 5.5%, and 5.7% of the recipients with renal vascular diseases, renal interstitial diseases/pyelonephritis/uropathy, diabetic renal disease, hereditary renal diseases, and diseases with unknown etiologies, respectively. Allograft survival was significantly decreased in patients with PTGN. PTGN was associated with a fourfold increase in graft failure with a hazard ratio of 7.11 for both acute rejection and PTGN. Results of the risk factor analysis for PTGN revealed that the underlying glomerular renal diseases and treatment methods using drugs such as tacrolimus and basiliximab significantly increased PTGN development, after adjusting for other risk factors.
Conclusion
We conclude that PTGN is strongly associated with poor kidney allograft survival. Therefore, optimal management of recurrent or de novo GN should be the critical focus of post-transplant care.
Keywords
Glomerulonephritis, Graft survival, Kidney transplantation, Risk factorsChronic glomerulonephritis (GN) is one of the main etiologies of end-stage renal disease (ESRD), and is an indication for kidney transplantation in 30–50% of the recipients with renal diseases [1]. Although much improvement in immunosuppressive regimens for the treatment of renal diseases has been achieved in the last 20 years, long-term allograft outcome has not significantly improved [2], [3], [4].
Along with the major impact of interstitial fibrosis, tubular atrophy, and chronic rejection on allograft outcome, recurrence or de novo development of GN (post-transplant glomerulonephritis or PTGN) may be one of the factors affecting long-term outcome [5], [6].
GN of all types may recur or develop soon after kidney transplantation and the prevalence of PTGN depends on the original underlying kidney disease. For example, the prevalence rate is 20–50% in membranoproliferative GN (MPGN) Type I, 80–100% in MPGN Type II, 20–50% in focal segmental glomerulosclerosis (FSGS), 13–50% in immunoglobulin A nephropathy (IgAN), and 10–30% in membranous nephropathy (MN) [7].
Our recent work highlights the significant effect of IgAN recurrence on allograft outcome, the results of which revealed that chronic changes negatively affected allograft outcome more significantly than IgAN recurrence [8]. Although the data are limited, the development of various types of PTGN and kidney allograft failure due to PTGN has been reported in patients with underlying chronic GN [9], [10], [11]. By contrast, many patients with chronic kidney disease with unknown etiology progress to ESRD and had to undergo renal transplantation; however, allograft outcome in these groups of patients has not been thoroughly investigated.
Therefore, we aimed to investigate and identify the incidence, risk factors, and the effect of PTGN on graft survival in both patients with chronic GN and patients with ESRD of unknown etiology.
We enrolled 764 of the 996 patients who underwent kidney transplantation at Seoul National University Hospital, Seoul National University Bundang Hospital, and Seoul National University Boramae Medical Center between 1995 and 2010. We excluded patients who were under 18 years of age and those who were retransplanted or underwent multiorgan transplantation. This study was approved by the Seoul National University Hospital Institutional Review Board. All clinical investigations were conducted in accordance with the guidelines set by the 2008 Declaration of Helsinki.
Patient gender, age, comorbidities, donor type, human leukocyte antigen (HLA) status, original kidney disease, date of referral, date of transplantation, and regimens of immunosuppressant drugs were recorded. Original kidney diseases were classified into six groups as follows: glomerular diseases, renal vascular diseases, renal interstitial diseases/pyelonephritis (PN)/uropathy, diabetic renal disease, hereditary renal diseases, and diseases with unknown etiologies. These diseases were diagnosed based on the results of kidney biopsy tests or imaging studies such as computed tomography, magnetic resonance imaging, and kidney ultrasonography, or based on clinical judgments by physicians and researchers. Early referral was defined as the interval between the nephrologist's visit and the diagnosis of ESRD or the start date of renal replacement therapy greater than 1 year.
Hypertension was defined as a systolic blood pressure greater than or equal to 140 mmHg, diastolic pressure greater than or equal to 90 mmHg, or the concurrent use of antihypertensive medications. Diabetes mellitus was diagnosed in patients with random blood glucose concentration levels greater than or equal to 200 mg/dL, fasting plasma glucose levels greater than or equal to 126 mg/dL on at least two separate occasions, or in patients using antihyperglycemic drugs.
Clinical parameters such as serum creatinine, estimated glomerular filtration rate (eGFR), hematuria, and daily proteinuria that could have influenced the development of PTGN while performing biopsy of the kidney were collected. Serum creatinine levels were measured using an assay based on Jaffe's method and eGFR was calculated using Modification of Diet in Renal Disease formula (MDRD): GFR (mL/minute/1.73 m2)=186×(Scr)1.154×(age in years)−0.203×(0.742 if female).
Biopsies of the allograft were performed when eGFR fell below 60 mL/minute or clinically significant hematuria (gross hematuria or red blood cell count ≥5/high power field in urinalysis and microscopy) or proteinuria [random urine protein-to-creatinine ratio (PCR) >0.5] developed. All protocol biopsies were excluded from the study. Acute rejection or PTGN was diagnosed by accurate histological classification based on the results of kidney biopsy. However, biopsy-unproven cases but with clinical indications that are mentioned above were not classified as PTGN. The date of diagnosis of PTGN was recorded as the date of biopsy. Graft failure was defined as the requirement for permanent dialysis or allograft nephrectomy, retransplantation, and censoring the recipient's death.
A standardized immunosuppression protocol involving a combination of a calcineurin inhibitor and steroids was initiated within 24 hours of surgery. The choice of calcineurin inhibitor, either cyclosporine A (CsA) or tacrolimus, was determined by the transplantation team. The initial dose of CsA was 10 mg/kg per day by the oral route; target trough levels were 200–400 ng/mL during the first 4 weeks and 100–200 ng/mL thereafter. The initial dose of tacrolimus was 0.16 mg/kg per day by the oral route; target trough levels were 8–15 ng/mL during the first 3 months and 3–8 ng/mL thereafter. Methylprednisolone (1 g/day) was administered by intravenous infusion on the day of transplantation, tapered to prednisone at 30 mg/day on post-transplantation day four, and then administered at the maintenance dose without withdrawal. Inhibitors of purine synthesis [e.g., mycophenolate mofetil (MMF)] were used in the initial immunosuppressive treatment based on clinical judgments considering risk factors such as HLA mismatch.
We compared the categorical variables using the Chi-square test and continuous variables expressed as the mean±standard deviation were compared using the Student t test or one-way analysis of variance. The Kaplan–Meier method was used to estimate the incidence rate of PTGN and graft failure as well as to compare cumulative probability of PTGN with several risk factors (log-rank test). The effect of PTGN on death-censored graft failure was also evaluated using Kaplan–Meier analysis. Cox proportional hazard models were used to examine the association between multiple risk factors and PTGN occurrence. While performing multivariate analysis, statistically significant covariates from the univariate analysis (P<0.01) were selected. A P value <0.05 was considered to be statistically significant. Statistical analysis was performed using SPSS version 18.0 software (SPSS Inc., Chicago, IL, USA).
Patient demographics are shown in Table 1. Mean duration of follow-up was 60.71±50.77 (range: 0.4–182.5) months. As underlying renal diseases, among overall 764 recipients, 292 (38.2%) had glomerular diseases, and 131 (17.1%), 45 (5.9%), 82 (10.7%), and 73 (9.6%) were classified to as having renal vascular diseases, renal interstitial diseases/PN/uropathy, diabetic renal disease, and hereditary renal diseases, respectively. The remaining 141 (18.5%) patients underwent kidney transplantation due to renal failure caused by unknown etiologies. Of the 292 patients in the glomerular diseases group, histological confirmation was performed in 195 (66.8%) patients. The results of which are as follows: 66.1% of the patients are with IgAN, 8.2% with FSGS, 7.2% with lupus nephritis, 4.1% with MPGN, 2.1% with MN, and 12.3% with other GN (Table 2).
Regarding donor type, living related donors were the most common, followed by deceased and living unrelated donors. A total of 569 (74.5%) patients were referred early to nephrologists. A total of 274 patients (35.9%) received pre-emptive transplantation. Tacrolimus and CsA were administered to 50.9% and 48.3% of recipients, while 42.1% received basiliximab pretransplantation. MMF was the most commonly used antimetabolite antirejection drug (75.1%).
During follow-up, 70 (9.2%) patients were diagnosed with PTGN. At diagnosis, serum creatinine level was 1.88±2.24 mg/dL, eGFR was 49.67±20.97 mL/minute, and random urine PCR was 2.57±3.18 g (Supplementary Table 1).
The incidence of PTGN increased with time after transplantation, from 4.9% at 2 years to 9.7% at 5 years, and 17.0% at 10 years of follow-up. PTGN occurred steadily throughout the follow-up period, while most acute rejection episodes developed early (Fig. 1).
Regarding underlying kidney diseases, PTGN was diagnosed in 17.8% of patients with glomerular diseases, compared with 0.0%, 4.4%, 4.9%, and 5.5% of the recipients in renal vascular diseases, renal interstitial diseases/PN/uropathy, diabetic renal disease, and hereditary renal diseases group, and in 5.7% of the patients with diseases of unknown etiologies (Table 2). Furthermore, in the glomerular diseases group, the incidence of PTGN increased more rapidly than in other groups (Fig. 3A). Among all types of PTGN, the most common type was IgAN (77.1%), followed by FSGS (8.6%) and MPGN (5.7%) (Table 2).
Allograft survival was markedly decreased in patients with PTGN (P<0.001) (Fig. 2A). When we analyzed graft survival by the incidence of PTGN and acute rejection, we found that as PTGN developed, the risk of graft failure increased by 4.02-folds [95% confidence interval (CI): 1.74–9.29, P=0.001]. In case of the occurrence of both acute rejection and PTGN, the hazard ratio (HR) of graft failure was 7.11 (95% CI: 2.96–17.07, P<0.001). After adjusting for other risk factors, PTGN was the strongest risk factor for graft failure (Table 3, Fig. 2B).
In addition, we identified graft survival by the incidence of PTGN, acute rejection, and chronic rejection including chronic allograft nephropathy (CAN). In any combinations of the development of acute rejection and CAN, as PTGN developed, the risk of graft failure increased to a considerable extent (Table 4). However, the number of patients with the occurrence of all of them was small, so HR of graft failure seems to be underestimated.
When analyzing the effects of several types of PTGN on allograft survival, there was no significant difference between IgAN, FSGS, MPGN, and immune-mediated GN, and this difference was also verified using Kaplan–Meier analysis (Supplementary Table 2, Supplementary Fig. 1).
Of the underlying kidney diseases, glomerular disease was a significant risk factor for PTGN (HR: 3.63, 95% CI: 1.73–7.65, P=0.001; Table 5, Fig. 3A). The incidence of PTGN increased in patients who had kidney transplantation since 2001, especially after 2006 (HR: 2.52, 95% CI: 1.16–5.46, P=0.019); however, after adjusting for other risk factors, this was not found to be statistically significant.
By contrast, there was no difference in PTGN development according to age, gender, donor type, timing of referral, and pre-emptive transplantation. HLA mismatch was also not significantly associated with the development of PTGN.
The use of antimetabolites such as MMF and azathioprine did not influence the development of PTGN. The administration of basiliximab before transplantation and the use of tacrolimus actually increased the incidence of PTGN, and in multivariate analysis, these results were found to be statistically significant (HR: 1.89, 95% CI: 1.08–3.32, P=0.027) (HR: 2.10, 95% CI: 1.25–3.54, P=0.005) (Table 4, Figs. 3B and 3C).
The aim of this study was to analyze PTGN incidence, its relationship with allograft failure, and the risk factors for PTGN development. PTGN increased over the duration of follow-up, reaching a cumulative probability of 17.0% after 10 years. The development of PTGN significantly decreased allograft survival. Among underlying kidney diseases, the highest occurrence of PTGN was in the glomerular diseases group, with the most common type being IgAN. Also, we demonstrated that PTGN significantly contributed to allograft dysfunction, and eventual graft loss, irrespective of the type of PTGN, and that PTGN incidence markedly increased in patients with baseline glomerular diseases and with the use of tacrolimus and basiliximab. We investigated the clinical courses and prognosis of patients, including PTGN and allograft survival in the unknown etiology group. In many previous studies, these outcomes were analyzed only in patients with pathologically confirmed or presumed glomerular diseases.
The results of our study are consistent with those of an earlier study, which reported that the incidence of PTGN among patients who underwent kidney transplantation after 2001 was significantly higher than those who underwent transplantation before 2001, and that the risk for allograft failure was definitely increased in patients with PTGN [12]. Because chronic transplant glomerulopathy confirmed by pathologists was excluded from the analysis of graft survival, we were able to verify the effect of only PTGN on allograft failure in this study.
PTGN developed steadily and consistently, and allograft loss due to PTGN increased throughout the follow-up period, while the incidence of allograft loss due to acute rejection was the highest 1 year after transplantation, but rapidly decreased thereafter [13]. This finding suggests that the current immunosuppression regimens do not influence the incidence and course of PTGN, similar to the findings reported by Briganti et al. [12], [13]. In addition, our findings are confirmed by the presence of pathologically confirmed glomerular diseases in the native kidney as a critical predictor of PTGN [13]. Also, this study verified the poorest graft survival in patients with both acute rejection and PTGN.
Moreover, the fact that administration of tacrolimus increased PTGN is confirmed by the results of a previous study in which recipients treated with tacrolimus instead of CsA had more frequent proteinuria and an increased risk of graft failure [14]. As proteinuria is a primary manifestation of GN, patients with PTGN have more proteinuria, interstitial change, and glomerular change, and eventually progress to allograft loss [15].
Among underlying glomerular diseases, FSGS recurred in 5 of the 16 patients (31.3%), while the MPGN and IgAN recurrence rate were 25.0% and 15.5%, respectively. These results were comparable with usual recurrence rates reported previously. By contrast, MN did not recur compared with the expected rate of 10–30%; it is assumed that this difference is due to demographic differences in the study population of our centers, and that the recurrence rate will increase with longer follow-up periods [7].
In addition, our findings differ from those of Karakayali et al. in that the recurrence rates were similar among patients taking either tacrolimus or CsA [16] and from those of Gaston et al. in that the recipients treated with tacrolimus had better kidney function than those on CsA-based treatment [17]. Furthermore, the results of our study differ from another study that documented an increasing tendency toward PTGN development in living related kidney transplants with a higher degree of HLA matching [18], [19].
Our study has a different level of significance compared with others, in that we demonstrated a positive correlation between the use of tacrolimus and basiliximab and PTGN. Even if better immunosuppression regimens decrease post-transplant complications, including treatment-resistant rejection and overall long-term mortality, prolonged graft survival and rejection-free survival were thought to contribute to the higher incidence of PTGN [20]. In addition, completeness of follow-up of the cohorts with the administration of tacrolimus and basiliximab might influence this result.
After adjusting for multiple factors such as increased numbers of kidney biopsies, advances in medicine, and more accurate interpretations of pathologic results, our results appear to be meaningful. It is noteworthy that a cohort of three centers in Asia became the subject of this study and the duration of follow-up was long enough to obtain an accurate interpretation of these results.
Also, even in cases of underlying diabetic renal diseases, PTGN occurred to a considerable extent accounting for 4.9%. It suggests that some patients with diabetes mellitus and renal failure may have underlying glomerular diseases, and therefore the patients without typical features and courses of diabetic renal diseases should have kidney biopsies taken to evaluate underlying glomerular diseases.
There were several limitations to our study. First, it was not possible to distinguish de novo glomerular diseases from recurrent glomerular diseases in a few patients, because pathologically unproven or only clinically diagnosed glomerular diseases were included in the underlying glomerular diseases group without clear criteria for classification, and 141 (18.5%) patients had diseases with unknown etiologies despite classifying the underlying kidney diseases into six groups. Also, some patients were categorized as having original renal diseases without definite criteria. In other words, true incidence and influence of PTGN is underestimated or overestimated in this group. Thus, we need to design a follow-up study with an accurate classification of de novo and recurrent GN. Second, biopsies of the allograft appeared to be performed more often in patients who underwent transplantation after 2001, regardless of the level of proteinuria (Supplementary Table 1). This could have contributed to an exaggerated effect on the analysis of results. Accordingly, verification of the effects of tacrolimus and basiliximab should be practiced through well-designed, multicentered, prospective and large cohort studies in the future.
It is expected that the number of kidney transplants will increase, and developing effective immunosuppression treatment methods will reduce early and late complications, and enhance comprehensive outcomes and survival rates after transplantation. In this light, the importance of PTGN will be emphasized. We demonstrated that PTGN has a prominent and considerable influence on allograft survival like acute rejection, even in transplant recipients with ESRD of unknown etiology. We suggest that understanding the risk factors and prognosis of PTGN should play a significant role in the monitoring and management of renal allograft patients in the future.
In conclusion, PTGN was strongly associated with poor kidney allograft survival. A critical focus of post-transplant care should be the management of recurrent or de novo GN.
Acknowledgments
Presented at Kidney Week 2011 of the American Society of Nephrology, Philadelphia, PA, November 8–13, 2011. Presented at American Transplant Congress 2012 of the American Society of Transplantation, Boston, MA, June 2–6, 2012. This work was supported by a grant from the Korean Healthcare Technology R&D Project, Ministry for Health, Welfare & Family Affairs, Republic of Korea (A084001).
Notes
Supplementary data associated with this article can be found in the online version at 10.1016/j.krcp.2012.09.004.
References
1. Choy BY, Chan TM, Lai KN. Recurrent glomerulonephritis after kidney transplantation. Am J Transplant 6:2006;2535–2542.


2. Golgert WA, Appel GB, Hariharan S. Recurrent glomerulonephritis after renal transplantation: an unsolved problem. Clin J Am Soc Nephrol 3:2008;800–807.



5. Hariharan S, Adams MB, Brennan DC, Davis CL, First MR, Johnson CP, Ouseph R, Peddi VR, Pelz CJ, Roza AM, Vincenti F, George V. Recurrent and de novo glomerular disease after renal transplantation: a report from Renal Allograft Disease Registry (RADR). Transplantation 68:1999;635–641.


6. Denton MD, Singh AK. Recurrent and de novo glomerulonephritis in the renal allograft. Semin Nephrol 20:2000;164–175.

7. O'Meara Y, Green A, Carmody M, Donohoe J, Campbell E, Browne O, Walshe J. Recurrent glomerulonephritis in renal transplants: fourteen years’ experience. Nephrol Dial Transplant 4:1989;730–734.


8. Han SS, Huh W, Park SK, Ahn C, Han JS, Kim S, Kim YS. Impact of recurrent disease and chronic allograft nephropathy on the long-term allograft outcome in patients with IgA nephropathy. Transpl Int 23:2010;169–175.


9. Hariharan S, Johnson CP, Bresnahan BA, Taranto SE, McIntosh MJ, Stablein D. Improved graft survival after renal transplantation in the United States, 1988 to 1996. N Engl J Med 342:2000;605–612.


10. Ostrowska J, Pazik J, Lewandowski Z, Mróz A, Perkowska-Ptasińska A, Durlik M. Posttransplantation glomerulonephritis: risk factors associated with kidney allograft loss. Transplant Proc 39:2007;2751–2753.


11. Briggs JD, Jones E. Recurrence of glomerulonephritis following renal transplantation. Scientific Advisory Board of the ERA-EDTA Registry. European Renal Association-European Dialysis and Transplant Association. Nephrol Dial Transplant 14:1999;564–565.

12. Chailimpamontree W, Dmitrienko S, Li G, Balshaw R, Magil A, Shapiro RJ, Landsberg D, Gill J, Keown PA; Genome Canada Biomarkers in Transplantation Group. Probability, predictors, and prognosis of posttransplantation glomerulonephritis. J Am Soc Nephrol 20:2009;843–851.



13. Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med 347:2002;103–109.


14. Sancho A, Gavela E, Avila A, Morales A, Fernández-Nájera JE, Crespo JF, Pallardo LM. Risk factors and prognosis for proteinuria in renal transplant recipients. Transplant Proc 39:2007;2145–2147.


15. Couser W. Recurrent glomerulonephritis in the renal allograft: an update of selected areas. Exp Clin Transplant 3:2005;283–288.

16. Karakayali FY, Ozdemir H, Kivrakdal S, Colak T, Emiroğlu R, Haberal M. Recurrent glomerular diseases after renal transplantation. Transplant Proc 38:2006;470–472.


17. Gaston RS. Current and evolving immunosuppressive regimens in kidney transplantation. Am J Kidney Dis 47(4 Suppl 2):2006;S3–S21.


18. Mathew TH. Recurrence of disease following renal transplantation. Am J Kidney Dis 12:1988;85–96.


Figure 1
Incidence rate of post-transplant glomerulonephritis (PTGN) compared with acute rejection. The incidence rate of PTGN increased steadily and consistently over the duration of follow-up, whereas acute rejection occurred rapidly in the early post-transplant period, and the slope decreased thereafter.
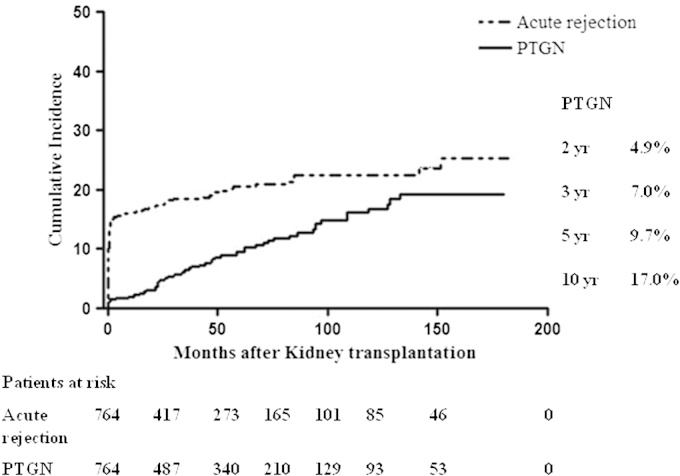
Figure 2
Effect of both post-transplant glomerulonephritis (PTGN) and acute rejection (AR) on graft survival. (A) Association between PTGN prevalence and graft failure. The development of PTGN was an obvious risk factor for kidney allograft failure. In patients with PTGN, graft survival decreased significantly (P<0.001). (B) Effects of AR and PTGN on graft failure. As PTGN developed, the incidence of graft failure significantly increased. Furthermore, the presence of both AR and PTGN caused kidney allograft failure to increase. *Patients without occurrence of both acute rejection and PTGN, †Patients with occurrence of PTGN, but without acute rejection, ‡Patients with occurrence of acute rejection, but without PTGN, §Patients with occurrence of both acute rejection and PTGN
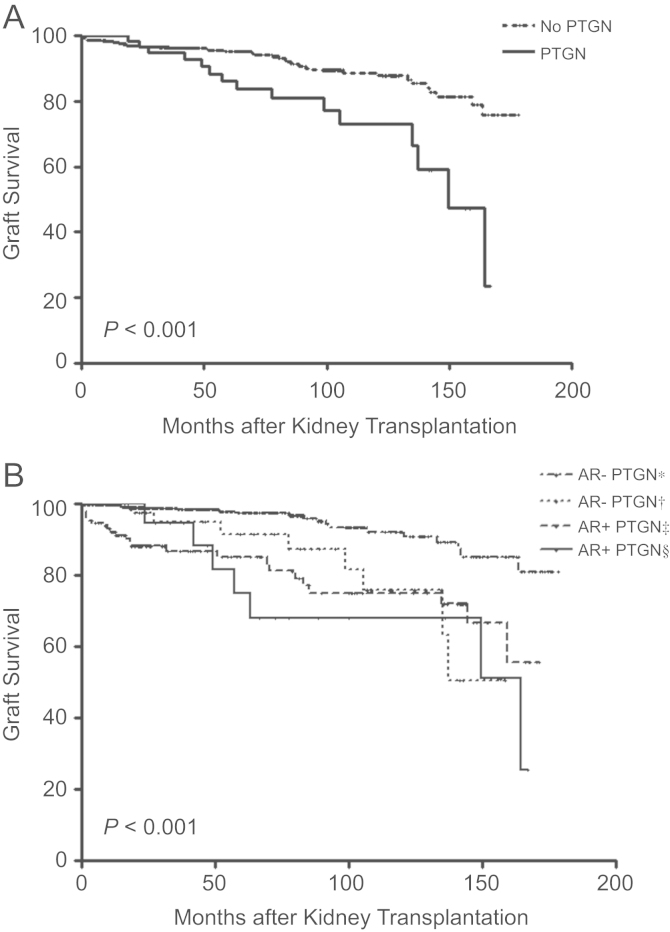
Figure 3
Incidence of post-transplant glomerulonephritis (PTGN) according to the influence of risk factors. (A) Association between underlying kidney disease and PTGN. In the glomerular diseases group, the incidence rate of PTGN is more than in other etiology groups (P<0.001). The significant difference between the glomerular diseases group and the unknown etiology group was specifically in the development of PTGN (P<0.001). In all groups tested, PTGN increased over the duration of follow-up. (B) Association between PTGN and the use of calcineurin inhibitors. The use of tacrolimus increased the PTGN development compared with cyclosporine (P=0.001). (C) Association between PTGN and the use of basiliximab. The use of basiliximab increased the PTGN development compared with no use of basiliximab (P=0.005). PN, pyelonephritis.
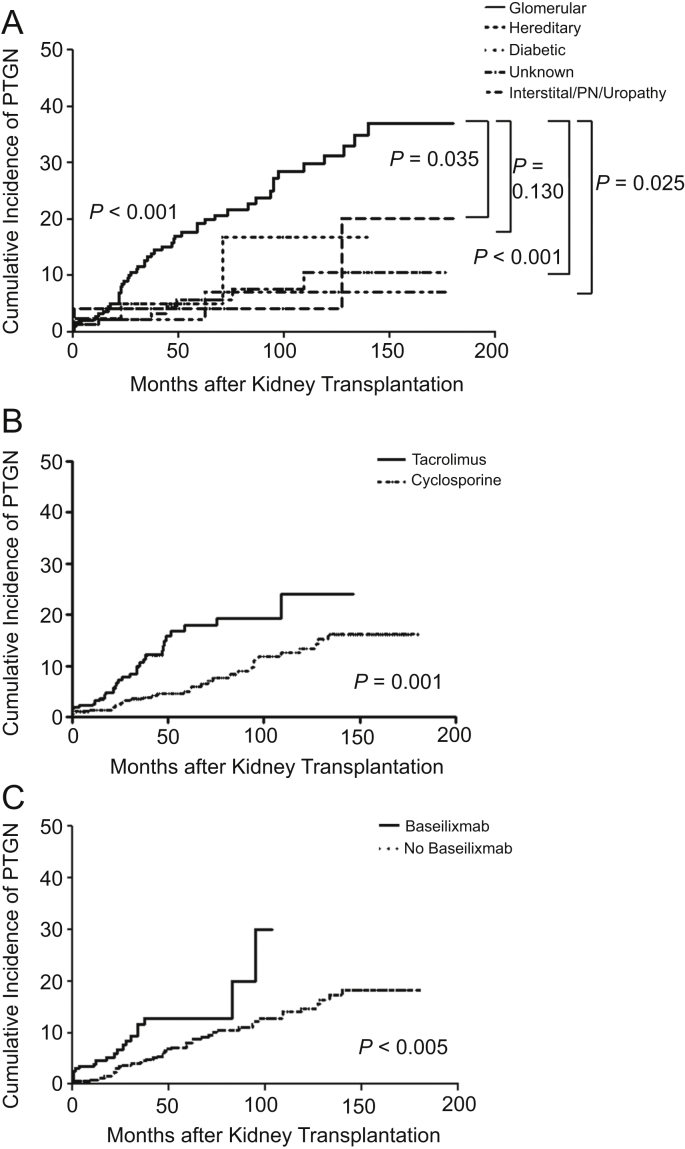
Table 1
Baseline characteristics classified by underlying kidney diseases
| Total (N=764) | Glomerular (N=292, 38.2%) | Vascular (N=131, 17.1%) | Interstitial/PN/Uropathy (N=45, 5.9%) | Diabetic (N=82, 10.7%) | Hereditary and various (N=73, 9.6%) | Unknown (N=141, 18.5%) | |
|---|---|---|---|---|---|---|---|
| Era | |||||||
| 1995–2000 | 173 (22.6) | 72 (24.7) | 33 (25.2) | 12 (26.7) | 4 (4.9) | 13 (17.8) | 39 (27.7) |
| 2001–2005 | 235 (30.8) | 89 (30.5) | 45 (34.4) | 15 (33.3) | 23 (28.0) | 19 (26.0) | 44 (31.2) |
| 2006–2010 | 356 (46.6) | 131 (44.8) | 53 (40.4) | 18 (40.0) | 55 (67.1) | 41 (56.2) | 58 (41.1) |
| HLA mismatch | |||||||
| Unknown | 22 (2.9) | 8 (2.7) | 0 (0) | 0 (0) | 4 (4.9) | 1 (1.4) | 9 (6.4) |
| 0 | 100 (13.1) | 44 (15.1) | 13 (9.9) | 10 (22.2) | 6 (7.3) | 7 (9.6) | 20 (14.2) |
| 1–3 | 410 (53.7) | 162 (55.5) | 77 (58.8) | 23 (51.1) | 40 (48.8) | 33 (45.2) | 75 (53.2) |
| 4–6 | 232 (30.3) | 78 (26.7) | 41 (31.3) | 12 (26.7) | 32 (39.0) | 32 (43.8) | 37 (26.2) |
| Male (recipient) | 446 (58.4) | 167 (57.2) | 83 (63.4) | 24 (53.3) | 55 (67.1) | 45 (61.6) | 72 (51.1) |
| Male (donor) | 406 (54.0) | 161 (56.1) | 77 (58.8) | 21 (46.7) | 42 (52.5) | 42 (57.5) | 63 (46.3) |
| Donor type | |||||||
| Living related | 451 (59.0) | 190 (65.1) | 75 (57.3) | 27 (60.0) | 43 (52.4) | 34 (46.6) | 82 (58.2) |
| Living unrelated | 141 (18.5) | 42 (14.4) | 35 (26.7) | 4 (8.9) | 18 (22.0) | 18 (24.7) | 24 (17.0) |
| Deceased | 172 (22.5) | 60 (20.5) | 21 (16.0) | 14 (31.1) | 21 (25.6) | 21 (28.7) | 35 (24.8) |
| Referral | |||||||
| Early (≥1 y) | 569 (74.5) | 249 (85.3) | 71 (54.2) | 41 (91.1) | 78 (95.2) | 70 (95.9) | 60 (42.6) |
| Late (<1 y) | 178 (23.3) | 38 (13.0) | 54 (41.2) | 4 (8.9) | 2 (2.4) | 3 (4.1) | 77 (54.6) |
| Unknown | 17 (2.2) | 5 (1.7) | 6 (4.6) | 0 (0) | 2 (2.4) | 0 (0) | 4 (2.8) |
| Pre-emptive | 274 (35.9) | 113 (38.7) | 50 (38.2) | 10 (22.2) | 22 (26.8) | 29 (39.7) | 50 (35.5) |
| Diabetes mellitus | 93 (12.2) | 3 (1.0) | 2 (1.5) | 1 (2.2) | 82 (100.0) | 3 (4.1) | 2 (1.4) |
| Hypertension | 523 (68.5) | 192 (65.8) | 131 (100.0) | 20 (44.4) | 58 (70.7) | 33 (45.2) | 89 (63.1) |
| Immunosuppressants | |||||||
| Calcineurin inhibitors | |||||||
| Cyclosporine A | 369 (48.3) | 137 (46.9) | 71 (54.2) | 20 (44.4) | 42 (51.2) | 27 (37.0) | 72 (51.1) |
| Tacrolimus | 389 (50.9) | 155 (53.1) | 58 (44.3) | 24 (53.4) | 38 (46.4) | 46 (63.0) | 68 (48.2) |
| Others | 6 (0.8) | 0 (0) | 2 (1.5) | 1 (2.2) | 2 (2.4) | 0 (0) | 1 (0.7) |
| Purine synthesis inhibitors | |||||||
| Not use | 82 (10.7) | 35 (12.0) | 17 (13.0) | 5 (11.1) | 2 (2.4) | 8 (11.0) | 15 (10.6) |
| MMF | 574 (75.2) | 215 (73.6) | 90 (68.7) | 34 (75.6) | 77 (93.9) | 56 (76.7) | 102 (72.3) |
| Azathioprine | 107 (14.0) | 41 (14.0) | 24 (18.3) | 6 (13.3) | 3 (3.7) | 9 (12.3) | 24 (17.1) |
| Others | 1 (0.1) | 1 (0.4) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Basiliximab | 322 (42.1) | 115 (39.4) | 50 (38.2) | 20 (44.4) | 46 (56.1) | 38 (52.1) | 53 (37.6) |
| Age at transplant⁎ | 41.49±11.97 | 38.69±11.06 | 46.18±10.60 | 35.22±12.71 | 52.10±9.18 | 39.84±13.43 | 39.65±10.46 |
| Donor age⁎ | 38.80±12.26 | 38.25±11.71 | 38.58±10.95 | 37.98±12.52 | 40.44±14.77 | 40.79±12.92 | 38.38±12.57 |
Table 2
Association between underlying kidney diseases and the incidence of PTGN
| Underlying kidney disease | N |
Patients with PTGN |
||||||
|---|---|---|---|---|---|---|---|---|
| IgAN | FSGS | MPGN | MN | Immune-mediated GN | Lupus nephritis | Incidence of PTGN (%)⁎ | ||
| Glomerular | 292 | 42 | 4 | 2 | 1 | 2 | 1 | 52 (17.8) |
| Biopsy proven | ||||||||
| IgAN | 129 | 19 | 1 | 0 | 0 | 0 | 0 | 20 (15.5) |
| FSGS | 16 | 4 | 1 | 0 | 0 | 0 | 0 | 5 (31.3) |
| MPGN | 8 | 0 | 0 | 1 | 0 | 1 | 0 | 2 (25.0) |
| MN | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Lupus nephritis | 14 | 0 | 0 | 0 | 0 | 0 | 1 | 1 (7.1) |
| Other GN | 24 | 0 | 1 | 1 | 0 | 0 | 0 | 2 (8.3) |
| Clinically diagnosed | 97 | 19 | 1 | 0 | 1 | 1 | 0 | 22 (22.7) |
| Vascular | 131 | 0 | 0 | 0 | 0 | 0 | 0 | 0 (0.0) |
| Interstitial/PN/Uropathy | 45 | 2 | 0 | 0 | 0 | 0 | 0 | 2 (4.4) |
| Diabetic | 82 | 3 | 1 | 0 | 0 | 0 | 0 | 4 (4.9) |
| Hereditary/various | 73 | 1 | 1 | 1 | 1 | 0 | 0 | 4 (5.5) |
| Unknown | 141 | 6 | 2 | 0 | 0 | 0 | 0 | 8 (5.7) |
| Total | 764 | 54 | 8 | 3 | 2 | 2 | 1 | 70 (9.2) |
Table 3
Effects of acute rejection and PTGN on graft failure
| N |
Univariate analysis |
Multivariate analysis⁎ |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Acute rejection–PTGN–† | 564 | Reference | Reference | ||
| Acute rejection–PTGN+‡ | 46 | 3.84 (1.68–8.77) | 0.001 | 4.02 (1.74–9.29) | 0.001 |
| Acute rejection+PTGN–§ | 132 | 4.79 (2.65–8.65) | <0.001 | 5.40 (2.94–9.93) | <0.001 |
| Acute rejection+PTGN+∥ | 22 | 6.22 (2.62–14.76) | <0.001 | 7.11 (2.96–17.07) | <0.001 |
Table 4
Effects of acute and chronic rejection, and PTGN on graft failure
| N |
Univariate analysis |
Multivariate analysis⁎ |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | ||
| Acute rejection–CAN–PTGN– | 536 | Reference | Reference | ||
| Acute rejection–CAN–PTGN+ | 34 | 3.90 (1.42–10.69) | 0.008 | 2.71 (0.87–8.47) | 0.087 |
| Acute rejection–CAN+PTGN– | 22 | 3.92 (1.30–11.79) | 0.015 | 4.05 (1.16–14.20) | 0.029 |
| Acute rejection–CAN+PTGN+ | 12 | 5.63 (1.63–19.39) | 0.006 | 5.13 (1.34–19.61) | 0.017 |
| Acute rejection+CAN–PTGN– | 98 | 3.85 (1.82–8.16) | <0.001 | 4.66 (2.03–10.68) | <0.001 |
| Acute rejection+CAN–PTGN+ | 14 | 9.68 (3.76–24.92) | <0.001 | 11.20 (3.81–32.90) | <0.001 |
| Acute rejection+CAN+PTGN– | 38 | 8.93 (4.27–18.66) | <0.001 | 9.40 (4.09–21.60) | <0.001 |
| Acute rejection+CAN+PTGN+ | 10 | 2.28 (0.30–17.21) | 0.426 | 3.62 (0.39–33.34) | 0.256 |
Table 5
Multiple risk factors for developing PTGN
|
Univariate analysis |
Multivariate analysis⁎ |
|||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Underlying kidney disease | ||||
| Unknown | Reference | Reference | ||
| Glomerular | 3.63 (1.73–7.65) | 0.001 | 4.01 (1.82–8.84) | 0.001 |
| Vascular | 0.00 | 0.953 | 0.00 | 0.953 |
| Interstitial/PN/Uropathy | 0.83 (0.18–3.91) | 0.815 | 0.84 (0.17–4.04) | 0.826 |
| Diabetic | 1.63 (0.49–5.46) | 0.428 | 1.88 (0.54–6.53) | 0.320 |
| Hereditary/various | 1.27 (0.38–4.23) | 0.695 | 1.23 (0.36–4.22) | 0.743 |
| Era | ||||
| 1995–2000 | Reference | |||
| 2001–2005 | 1.76 (0.94–3.31) | 0.080 | ||
| 2006–2010 | 2.52 (1.16–5.46) | 0.019 | ||
| HLA mismatch | ||||
| 0 | Reference | |||
| 1–3 | 0.86 (0.43–1.74) | 0.680 | ||
| 4–6 | 1.04 (0.49–2.23) | 0.917 | ||
| Age at transplant (y) | ||||
| 1st percentile | Reference | |||
| 2nd percentile | 0.84 (0.46–1.53) | 0.566 | ||
| 3rd percentile | 0.60 (0.30–1.23) | 0.165 | ||
| 4th percentile | 0.82 (0.43–1.59) | 0.564 | ||
| Donor age (y) | ||||
| 1st percentile | Reference | |||
| 2nd percentile | 0.58 (0.31–1.09) | 0.090 | ||
| 3rd percentile | 0.62 (0.32–1.21) | 0.160 | ||
| 4th percentile | 0.62 (0.32–1.20) | 0.156 | ||
| Male gender (recipient) | 0.74 (0.46–1.19) | 0.211 | ||
| Male gender (donor) | 1.09 (0.67–1.75) | 0.734 | ||
| Donor type | ||||
| Living related | Reference | |||
| Living unrelated | 1.08 (0.57–2.05) | 0.823 | ||
| Deceased | 1.25 (0.69–2.26) | 0.468 | ||
| Referral | ||||
| Late referral | Reference | |||
| Early referral | 0.84 (0.50–1.41) | 0.510 | ||
| Pre-emptive transplantation | 0.59 (0.35–1.02) | 0.058 | ||
| Immunosuppressant | ||||
| Purine synthesis inhibitors | ||||
| Not used | Reference | |||
| Azathioprine | 0.49 (0.22–1.09) | 0.079 | ||
| Mycophenolate mofetil | 1.27 (0.65–2.47) | 0.489 | ||
| Calcineurin inhibitor | ||||
| Cyclosporine A | Reference | Reference | ||
| Tacrolimus | 2.26 (1.37–3.72) | 0.001 | 2.10 (1.25–3.54) | 0.005 |
| Basiliximab | 2.05 (1.20–3.52) | 0.009 | 1.89 (1.08–3.32) | 0.027 |
- TOOLS
-
METRICS

- Related articles
-
A Case of Crescentic Glomerulonephritis Superimposed on Membranous Glomerulopathy2010 March;29(2)
A Case of Membranous Glomerulonephritis in a Patient with HIV Infection2007 November;26(6)
Membranoproliferative glomerulonephritis in a patient with chronic brucellosis2018 ;37(3)
Primary glomerulonephritis: A review of important recent discoveries2013 September;32(3)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















