| Kidney Res Clin Pract > Volume 31(4); 2012 > Article |
|
Abstract
Ethylene glycol (EG) is a sweet-tasting, odorless organic solvent found in many agents, such as anti-freeze. EG is composed of four organic acids: glycoaldehyde, glycolic acid, glyoxylic acid and oxalic acid in vivo. These metabolites are cellular toxins that can cause cardio-pulmonary failure, life-threatening metabolic acidosis, central nervous system depression, and kidney injury. Oxalic acid is the end product of EG, which can precipitate to crystals of calcium oxalate monohydrate in the tubular lumen and has been linked to acute kidney injury. We report a case of EG-induced oxalate nephropathy, with the diagnosis confirmed by kidney biopsy, which showed acute tubular injury of the kidneys with extensive intracellular and intraluminal calcium oxalate monohydrate crystal depositions.
Keywords
Acute kidney injury, Calcium oxalate, Ethylene glycol, Metabolic acidosisEthylene glycol (EG) is a colorless, odorless, and soluble chemical agent, mainly used in automobile anti-freeze, brake solution and as an industrial solvent [1]. The ingestion of EG-containing solutions leads to severe morbidity and mortality worldwide [2], [3]. EG poisoning can result in acute kidney injury (AKI), requiring hemodialysis to restore kidney function [2]. The cause of the kidney injury is unknown, but is associated with tubular cell necrosis and EG metabolism. The end product, oxalic acid, has been linked with renal toxicity, when calcium oxalate monohydrate (COM) crystals precipitate into the tubular lumen [2], [3], [4].
A 42-year-old male was referred to the emergency department, due to altered mentality. He had a history of alcohol abuse. For the purpose of self-injury, he swallowed 500 mL of anti-freeze solution at 2 PM one afternoon. He was discovered 18 hours later, with a deteriorated mental status, and was transferred to the Konkuk Medical Center. When he was discovered, an empty bottle of anti-freeze solution was found, which was confirmed by an emergency medicine specialist.
On admission, the patient presented with a deteriorated mental status and looked acutely ill. His vitals were: blood pressure=150/90 mmHg, heart rate=72 beats/minute, respiratory rate=30 breaths/minute and temperature=36.4 °C. No specific finding was observed during physical examinations.
Blood tests on admission showed: WBC=29,440/mm3 (neutrophils 67%, lymphocytes 21%, monocytes 6%), hemoglobin=18.1 g/dL, and platelets=335,000/mm3. An arterial blood gas analysis showed: pH=6.85, PaCO2=11.1 mmHg, PaO2=149.4 mmHg, and bicarbonate=1.9 mmol/L (Table 1). Serum chemistry showed: sodium=144 mEq/L, potassium=5.2 mEq/L, chloride=102 mEq/L, calcium=8.0 mg/dL, phosphorus=8.2 mg/dL, glucose=218 mg/dL, BUN=13.2 mg/dL, creatinine=1.78 mg/dL, creatinine kinase=166 U/L and lactate dehydrogenase=1346 IU/L. Urinalysis results showed: specific gravity=1.010, pH=5.0, protein=2+, RBC=3+ (5–9/HPF) and a trace of calcium oxalate crystals. The calculated anion gap was 40.1 mEq/L, and the serum osmolar gap was 67.3 mOsm/kgH2O.
On admission, an endotracheal tube was inserted, to promote stable respirations and prevent aspiration caused by vomiting. Normal saline solution and sodium bicarbonate were administered to increase the extracellular volume. Ethanol was not administered, because a significant amount of time had elapsed since the intake of the poison. Ventilator care was provided once the patient was moved to the ICU. Despite the continuous administration of saline and sodium bicarbonate, metabolic acidosis was not improved, therefore, hemodialysis was performed 6 hours after admission. Hemodialysis was performed via a dual lumen catheter, at a blood flow rate of 250 mL/minute for 8 hours. Upon completion of hemodialysis, the results of the arterial blood gas analysis were pH=7.45, PaCO2=35.7 mmHg, PaO2=94.9 mmHg, and bicarbonate=24.0 mmol/L, with the anion gap=21.0 mEq/L, and the osmolar gap=2.6 mOsm/kgH2O (Table 1). On the 2nd day of admission, the patient reverted to an alert mental status and the ventilator was removed. His urine output was maintained at 30∼40 mL/hour and findings of metabolic acidosis and, electrolyte disturbances were markedly improved without further dialysis therapy.
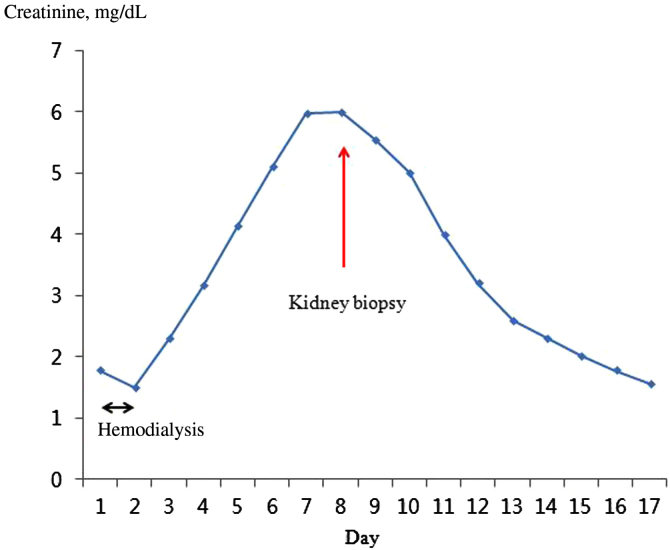
On the 6th day of admission, levels of BUN and creatinine had increased to 29.5 mg/dL and 6.0 mg/dL, respectively (Fig. 1). In order to elucidate the etiology of kidney injury, a kidney biopsy was performed, which showed typical deposition of COM crystals in the proximal tubule (Fig. 2). On the 9th day of admission, more than 100 mL/hour of urine output was maintained, and the serum creatinine level steadily improved (Fig. 1). On the 17th day of admission, levels of BUN and creatinine were 11.0 mg/dL and 1.57 mg/dL, respectively. The patient was discharged without any symptoms of toxicity of the central nervous system or cardiovascular system.
EG intoxication can be life-threatening, but early diagnosis and aggressive treatment are essential to prevent permanent disability or death [1], [8]. Most of the absorbed EG is metabolized in the liver (80%), and the remaining (20%) is excreted by the kidney without being metabolized [2], [4]. EG itself has weak toxicity, but its metabolites have strong toxicity. In the liver, alcohol dehydrogenase oxidizes EG to glycoaldehyde, and glycoaldehyde is transformed to glycolate, by aldehyde dehydrogenase. Symptoms of intoxication and high anion gap metabolic acidosis, which present 12–48 hours after intake, are mainly due to glycolate [2]. Transformation from glycolate to glyoxylate and oxalate takes a long time and accordingly, metabolic acidosis is aggravated, and cardiopulmonary dysfunction may occur. Oxalate, the end-product, reacts with calcium to form COM crystals, which are deposited in the brain, kidney, pericardium, liver and blood vessels, resulting in tissue damage. This tissue damage is mostly reversible, however, in some cases, damage results in permanent impairments [4], [9]. An antidote, fomepizole (4-methylpyrazole) or ethanol, competitively binds to alcohol dehydrogenase and is used to prevent the metabolism of EG to its toxic by-products. In most cases, however, EG metabolism has occurred before diagnosis, either because of the delay in getting to hospital or difficulties in making the diagnosis [2], [4].
For a definite diagnosis of EG poisoning, measurement of the EG concentration level is necessary, but practically difficult in most hospitals. Since rapid diagnosis and treatments are closely associated with the prognosis of the patients, clinical and laboratory findings are important for treating EG poisoning [1], [2], [3]. EG poisoning is suspected when there is the presence of high anion gap metabolic acidosis, a high osmolar gap, hypocalcemia, and crystalluria. However, the osmolar gap may not be increased in accordance with the metabolism of EG. Therefore, the possibility of EG poisoning cannot be excluded, even if the osmolar gap is normal [3]. In the urinalysis, calcium monohydrate crystals or calcium dihydrate crystals may be observed. Since these two types of hydrogenated crystals are present in plant tissues, detection in the urine does not necessarily mean EG poisoning [1].
In the present case, the patient was admitted about 18 hours after the intake of>1.4 mL/kg EG, which was a lethal dose. The serum EG level was not measured, because it takes a long time. On admission, the patient showed findings suggesting EG poisoning such as deterioration of the central nervous system, high anion gap metabolic acidosis with a high osmolar gap. After hemodialysis, acidosis was corrected within 24 hours and subsequent systemic improvements were accomplished, despite late presentation to the hospital. However, 1 week after admission, progressively deteriorating kidney function was observed (Fig. 1) with a urine output of 0.4∼0.5 mL/kg/hour. In many cases, EG intoxication is followed by kidney injury, like in this case [1], [2], [8], [10]. Although this case showed rather typical manifestations of EG intoxication, we did not test EG levels, so we felt it was necessary to perform a kidney biopsy to make a definite etiologic diagnosis of metabolic acidosis with AKI. Also, the patient was found relatively late after EG ingestion, so we could not exclude other possible causes of AKI such as volume depletion or ischemia. The characteristic finding of the kidney biopsy specimen was moderate acute tubular injury, with extensive intracellular and intraluminal crystal deposition. Hematoxylin and eosin staining showed translucent colorless refractile crystals in the destroyed tubules (Fig. 2A). Multi-colored birefringence and various forms of crystals were observed under the polarized light microscopic examination (Fig. 2B) [8].
The role of various EG metabolites (glycolate, glyoxylate, and oxalate) in producing the kidney injury have been widely debated over the years [11], [12]. Recent studies in rats have demonstrated that necrotic damage occurs only in the presence of COM and that the degree of damage correlates with the total accumulation of COM [2], [4], [9], [12]. COM accumulates in the kidney by attachment of crystals to tubular cell membranes, followed by the internalization by endocytosis. The internalization of COM by cells results in structural damage in cell membranes, production of free radicals and lipid peroxidation, malfunction in the mitochondria of proximal tubule cells and consequently, renal tubular necrosis and apoptosis [4], [11].
There are a few reports of EG poisoning in Korean literature, but this is the first report that includes a kidney biopsy finding that showed the typical pathologic change of oxalate nephropathy [5], [6], [7].
In the present case, we performed a kidney biopsy to exclude other possible cause of AKI and to make a definite diagnosis of AKI. If the cause of AKI is oxalate nephropathy showing deposition of COM crystals, preservative treatments should be maintained with the expectation of kidney function recovery. The development of a pharmacological approach to reduce COM adherence to tubular cells, and its cellular interactions, would be valuable, as this would decrease the renal toxicity in late treated cases of EG poisoning. Further studies on this approach may be necessary in the future.
Refererences
2. Ting SM, Ching I, Nair H, Langman G, Suresh V, Temple RM. Early and late presentations of ethylene glycol poisoning. Am J Kidney Dis 53:2009;1091–1097.


3. Soghoian S, Sinert R, Wiener SW, Hoffman RS. Ethylene glycol toxicity presenting with non-anion gap metabolic acidosis. Basic Clin Pharmacol Toxicol 104:2009;22–26.


4. McMartin K. Are calcium oxalate crystals involved in the mechanism of acute renal failure in ethylene glycol poisoning? Clin Toxicol (Phila) 47:2009;859–869.


5. Bae JO, Kang SG, Lim SM, Lee EY, Cho SG, Kim JH, Lee KY. A case of ethylene glycol poisoning with metabolic acidosis treated with hemodialysis. Korean J Nephrol 24:2005;1039–1043.
6. Kim SU, Lee DH, Moon SJ, Seo YS, Kim JS, Kang SW, Choi KH, Lee HY, Han DS, Kim BS. A case of acute renal failure, acute pancreatitis and delayed recovery of bone marrow suppression, accompanied with ethylene glycol intoxication. Korean J Nephrol 25:2006;159–163.
7. Yoon SJ, Chang WK, Song MJ, Hwang NC, Lim SJ, Paik WH, Kim YK, Kim SY, Kim YJ, Park BY, Cho MK, Lee GJ. Acute renal failure and delayed neuropathy due to anti-freeze ingestion. Korean J Med 61:2001;64–70.
8. Takahashi S, Kanetake J, Kanawaku Y, Funayama M. Brain death with calcium oxalate deposition in the kidney: Clue to the diagnosis of ethylene glycol poisoning. Leg Med (Tokyo) 10:2008;43–45.


9. Guo C, Cenac TA, Li Y, McMartin KE. Calcium oxalate, and not other metabolites, is responsible for the renal toxicity of ethylene glycol. Toxicol Lett 173:2007;8–16.


10. Guo C, McMartin KE. The cytotoxicity of oxalate, metabolite of ethylene glycol, is due to calcium oxalate monohydrate formation. Toxicology 208:2005;347–355.


Figure 2
Representative images of oxalate crystals in the renal tubules. (A) Arrows indicate translucent colorless refractile crystals in the destroyed tubule (H & E, × 400). (B) Multicolored birefringence and various forms of the calcium oxalate monohydrate crytals are shown (polarized light microscopic examination, × 400).
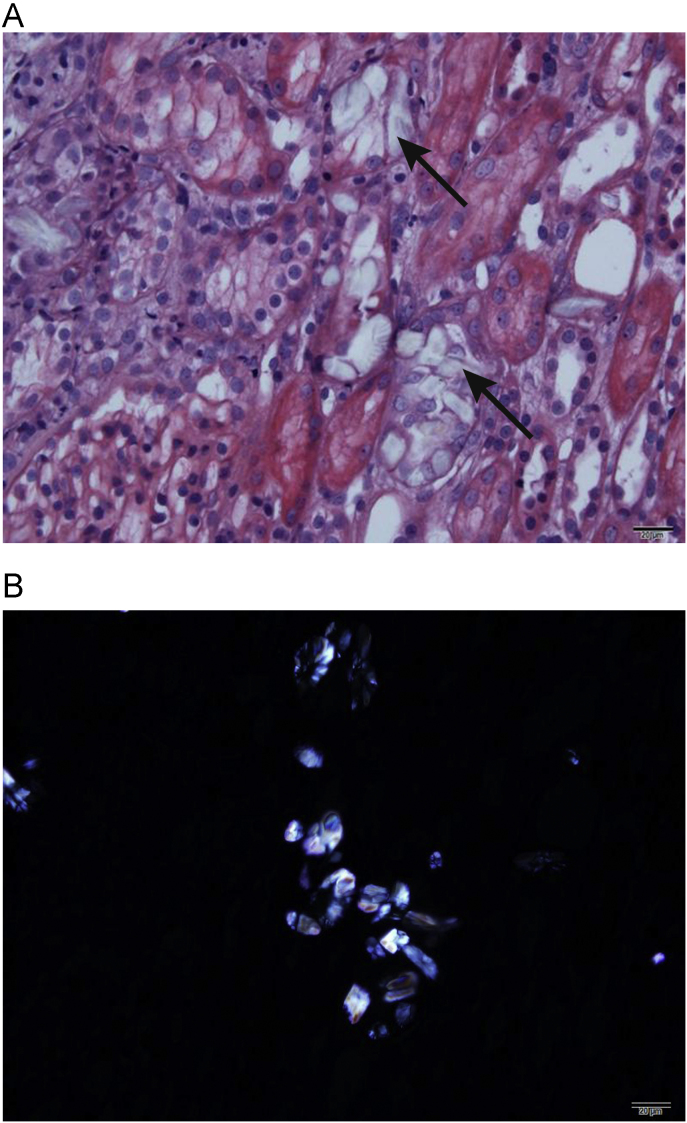
Table 1
Laboratory findings
| Time |
Arterial blood gas |
AG⁎(mEq/L) | OG†(mOsm/kgH2O) |
Chemistry |
|||||
|---|---|---|---|---|---|---|---|---|---|
| pH | PaCO2 (mmHg) | PaO2 (mmHg) | BE (mmol/L) | HCO3 (mmol/L) | BUN(mg/dL) | Cr(mg/dL) | |||
| On admission | 6.89 | 11.1 | 149.4 | –30.8 | 1.9 | 40.1 | 67.3 | 13.2 | 1.78 |
| After 5 h in hospital‡ | 7.14 | 14.9 | 177.2 | –21.7 | 4.9 | 33.1 | 52 | 14.5 | 1.76 |
| After 16 h in hospital§ | 7.45 | 35.7 | 94.9 | 0.5 | 24.0 | 21 | 2.6 | 6.0 | 1.11 |
| After 24 h in hospital | 7.45 | 33.9 | 105.2 | –0.6 | 22.8 | 20 | 1.5 | 8.0 | 1.51 |
| After 3 d | 7.44 | 36.9 | 62.0 | 1.0 | 24.7 | 15 | 1 | 15.9 | 3.17 |
| After 4 d | 7.41 | 29.6 | 83.0 | –4.0 | 19.0 | 15 | 2.6 | 19.9 | 4.14 |



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















