Outcomes of the surgical management of encapsulating peritoneal sclerosis: A case series from a single center in Korea
Article information
Abstract
Background
Encapsulating peritoneal sclerosis (EPS) is a rare but near-fatal complication of peritoneal dialysis (PD). Despite the high mortality rate of EPS, the surgical treatment strategy of severe EPS is yet to be established.
Methods
We retrospectively analyzed outcomes of patients with EPS who underwent enterolysis for intractable EPS at Seoul National University Hospital between 2001 and 2018. EPS was diagnosed based on the clinical symptoms and radiological findings of abdominal computed tomography (CT). CT scans were scored according to an EPS scoring system that assessed peritoneal thickening and calcification as well as bowel thickening, tethering, loculation, and dilatation.
Results
Thirteen patients (nine males and four females; age, 48 [29–63] years) underwent enterolysis for severe EPS. PD duration (11 [6–21] years) was not associated with survival. Two patients were newly diagnosed with EPS following kidney transplantation. Five patients died of infectious complications immediately after the surgery. Eight patients survived after the first surgery; however, five of them underwent reoperation but died of persistent infection, fistula formation, or adhesive bowel obstruction. Four young (< 60 years) male patients with relatively low CT scan scores (< 13) survived for > 2 years after the first surgery. Median survival duration from EPS diagnosis was 22 (1.3–184) months and that from the first surgery was 9 (0.3–153) months.
Conclusion
The high mortality rate of EPS suggests the importance of appropriate surgical intervention in young symptomatic male EPS patients with relatively low CT scan scores.
Introduction
Peritoneal dialysis (PD) is a standard therapy for end-stage renal disease (ESRD) [1]. PD shows several benefits, such as low annual medical costs, survival advantage over in-center hemodialysis in the early stage, and improved quality of life in terms of the convenience of home care [2]. However, with increased cumulative duration of PD, undesirable long-term complications, such as recurrent peritonitis, dialysis failure, and peritoneal sclerosis, develop.
Although very rare, encapsulating peritoneal sclerosis (EPS) is the most severe complication of long-term PD [3,4]. EPS is a syndrome that repeatedly manifests with partial or complete intestinal obstruction caused by encapsulating fibrotic tissues forming a cocoon-like structure with diffuse peritoneal thickening and calcification [5–7]. EPS in PD patients is a progressive disease with chronic intra-abdominal inflammation triggered by the iatrogenic PD solution. Symptomatic EPS often leads to PD discontinuation; however, it typically manifests after transfer to hemodialysis or kidney transplantation [6,8,9]. Clinically, this syndrome is characterized by obstructive symptoms, such as nausea, vomiting, abdominal pain, and malnutrition due to poor oral intake. These symptoms usually progress insidiously, and full-blown symptoms from bowel obstruction result in malnutrition and poor quality of life.
The overall incidence of EPS in the PD population is between 0.5% and 7.5% [8–13], which increases proportionally with PD duration, particularly of > 5 years. A prospective Japanese study reported that a 0% incidence of EPS 3 years after PD initiation increased to up to 17.2% after 15 years [8]. Once symptomatic EPS is diagnosed, the prognosis is substantially poor, with the mortality rate being up to 55% in the first year after diagnosis [13].
The pathophysiological mechanism underlying PD-associated EPS is explained by the “two-hit hypothesis” [10,13,14]. The first hit is triggered by predisposing factors such as PD initiation and repeated peritoneal exposure to the PD solution. Repeated PD cycles deteriorate peritoneal inflammation, fibrosis, and diffuse sclerosis. The second hit includes bacterial peritonitis, recurrent peritonitis, excessive peritoneal fibrogenetic reaction to the PD solution (which promotes transforming growth factor-β1 signaling cascades [15]), and elevated collagenosis and fibrogenesis [16].
Desperate therapeutic options have been employed to reverse the deteriorated clinical outcomes of EPS. Timely and early administration of steroids and tamoxifen before the development of advanced malnutrition and symptomatic EPS reduces intraperitoneal inflammation and ascites and relieves bowel obstructive symptoms [8,17,18]. However, although reports of successful therapeutic experiences in advanced EPS are relatively limited, successful surgical treatment of advanced EPS has been reported. Kawanishi et al [19] described 17 years of experience of surgical interventions for EPS and reported favorable outcomes in 181 of the 239 surgical treatments. Ulmer et al [20] reported successful surgical outcomes in 37% of the 45 EPS patients. However, a treatment strategy for the surgical management of severe EPS has not been established. Therefore, in this study, we aimed to describe our surgical experiences of advanced EPS in a single center in Korea.
Methods
Patients
From January 2001 to December 2018, 21 patients were diagnosed with EPS at Seoul National University Hospital. Thirteen patients underwent surgical intervention for intractable EPS. Surgery was considered for EPS treatment in patients who met any of the following conditions: development of intractable obstructive symptoms refractory to medication and conservative treatment, severe malnutrition despite long-term total parenteral nutrition, postprandial vomiting, and severe abdominal pain due to bowel obstruction, as documented by small bowel series or computed tomography (CT). All surgeries were performed by the same surgeon without a major change in the technique. We defined the initial mortality group as the deceased patients after the first EPS surgery and the initial survivor group as the survived patients after the 1st surgery.
All patients presented a history of PD preoperatively. EPS was diagnosed based on the guidelines proposed by the International Society for PD (ISPD) [5]. All patients presented with bowel obstructive symptoms, and contrast-enhanced abdominal CT confirmed encapsulated intestine and peritoneal and bowel wall thickening. The study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Institutional Review Board (IRB) of Seoul National University Hospital (approval number: H-1901-156-1006). The written informed consent from the participants for this retrospective study was waived by IRB.
Data collection
Patient data were retrospectively collected from the hospital’s electronic medical records. Demographic characteristics included age, sex, body mass index, primary ESRD cause, PD duration, PD discontinuation cause, and cumulative number of peritonitis episodes. Clinical symptoms related to EPS; laboratory findings during the perioperative period (serum albumin, hemoglobin, C-reactive protein, total calcium, and phosphorus levels); and cultured organisms during the individual episodes of peritonitis were reviewed. Moreover, CT findings, surgical records, postoperative progress, and outcomes were evaluated. CT scans were examined by experienced radiologists. CT scan scores were calculated according to a previously published scoring system [21]. Briefly, EPS progression was evaluated based on six scoring parameters: peritoneal thickening and calcification as well as bowel wall thickening, tethering, loculation, and dilatation. Each parameter was scored from 0 to 4 points (0–3 points for bowel tethering and loculation) according to pathological changes (Supplementary Table 1, available online) [21]. Bowel tethering was defined as an abrupt change in bowel caliber caused by severe fibrosis.
Results
Patient characteristics
Of the 21 patients diagnosed as having EPS, eight did not undergo surgical treatment. Of the eight, five patients presented with mild EPS symptoms, such as dyspepsia, decreased oral intake, and constipation, and they did not show severe obstructive symptoms requiring total parenteral nutrition. Among the five patients, kidney transplantation was performed in one and dialysis modality was changed to hemodialysis in four; however, one patient died of peritonitis-associated sepsis.
Thirteen patients (nine males and four females) underwent surgical treatment, and their baseline characteristics are described in Table 1. Median patient age was 48 (29–63) years. The underlying cause of renal disease was glomerulonephritis in five patients, hypertension in three, and other disorders in five; no patient had diabetes. Median PD duration was 11 (6–21) years. The most common cause of PD withdrawal was bacterial peritonitis (46.1%), followed by EPS (30.8%) and kidney transplantation (23.1%). Mean body mass index was 19.3 ± 2.4 kg/m2; mean serum albumin level was 3.1 ± 0.5 g/dL; and mean hemoglobin level was 10.1 ± 1.4 g/dL (Table 2). Pathogens were detected during prior episodes of peritonitis in most patients, with Enterococcus faecium being the most common pathogen.
Characteristics of EPS and surgical treatment
Most patients with EPS were diagnosed after PD discontinuation, and the median time from EPS development to surgery was 8 (0–31) months. All patients presented with severe obstructive symptoms (nausea, vomiting, and abdominal pain). Although some patients received total parenteral nutritional support, their symptoms and malnutrition did not improve. All patients underwent enterolysis or adhesiolysis as the first surgical intervention. However, some patients who required a second adhesiolysis underwent additive procedures. Case 2 underwent colostomy due to bowel perforation, case 5 underwent small bowel resection, case 6 underwent transverse colon resection, and case 8 underwent jejunostomy. Intraoperative and radiological findings before and after enterolysis are shown in Fig. 1. Abdominal CT scans of the EPS patients are shown in Fig. 2, demonstrating diffusely thickened and calcified peritoneal wall, dilated and thickened bowel loops, and loculated ascites. Table 2 summarizes the total CT scan scores. The median total CT score was 14 (6–17) (Table 2). The histologic finding of adhesiolysed peritoneum showed thickened peritoneal membrane, chronic inflammation, massive peritoneal fibrosis, and calcification (Fig. 3).
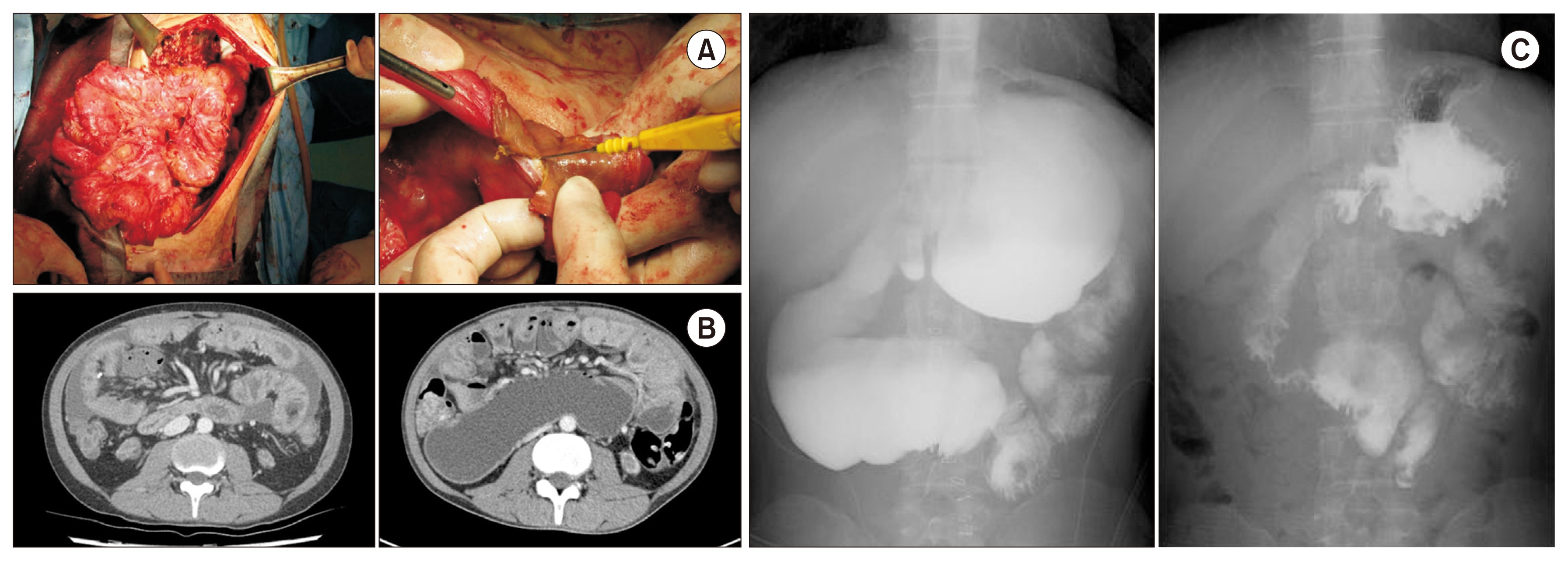
Intraoperative and radiological findings of encapsulating peritoneal sclerosis.
(A) The clumped intestine was covered with dense fibrosclerotic materials forming a cocoon-like structure (left). The dense, thick fibrotic cover was incised using a sharp knife during enterolysis (right). (B) Abdominal computed tomography showed thickened peritoneal and bowel walls, dilated and thickened bowel loops, and loculated ascites. (C) Small bowel series with gastrografin showed partial obstruction and delayed emptying of the small bowel from the stomach (left). However, this obstruction was released after laparotomy and adhesiolysis (right).

Contrast-enhanced computed tomography (CT) findings of advanced encapsulating peritoneal sclerosis.
Diffuse peritoneal calcification (filled arrows) and dilated and thickened bowel loops were noted on CT of Patient 1 (A) and Patient 10 (B). The thickened bowel wall and loculated ascites were also noted (asterisks). (C) Thickened peritoneal wall (arrowhead) and thickened bowel wall enclosed by the peritoneum (empty arrow) were noted.

Pathological findings of peritoneal specimen after surgery.
Hematoxylin and eosin (H&E) staining demonstrated a thickened peritoneal membrane (empty arrow), with chronic inflammation (asterisk), fibrous tissue (white arrows), and dystrophied calcification (black arrowheads) in the peritoneal specimen. (A) ×40, (B) ×100, and (C) ×200.
Outcomes and prognostic factors associated with EPS
Five patients died because of uncontrolled peritonitis and sepsis during postoperative care of the first surgery (Table 3). Eight patients survived after the first enterolysis. Median survival duration after the first surgery was 14 (1.3–27) months in the initial mortality group and 26.5 (6–184) months in the initial survivor group.
Five of eight survivors underwent reoperation for persistent peritonitis due to small bowel perforation (two patients), peritoneal obstruction and perforation (one patient), peritoneal abscess and small bowel obstruction (one patient), and enterocutaneous and internal fistula (one patient). Among these patients, cases 9 and 10 underwent adhesiolysis at the point of EPS diagnosis and survived for > 12 months after the first surgery; however, they underwent colostomy for small bowel perforation or obstruction and finally died because of systemic infection. All five patients who underwent reoperation died. Meanwhile, three of eight patients who survived after the first surgery have survived without reoperation until now. Cases 12 and 13 survived for > 5 years without any symptom, and case 11 survived for 2 years postoperatively. These three patients showed a good nutritional status on their last visit to the outpatient clinic.
The overall patient survival rate was 23.1%. Median survival durations from EPS diagnosis was 22 (1.3–184) months and that from the first surgery was 9 (0.3–153) months. There were no significant differences in CT scan scores between the initial mortality and initial survivor groups after the first surgery. However, the CT scan scores were < 13 in patients who survived for > 2 years after the first surgery (cases 10–13). CT scan scores of three patients (case 11–13), who showed the best postoperative performance, were ≤ 10, whereas the CT scan score of case 10, who maintained a relatively good performance for 30 months and died 32 months after the first surgery, was 12. Interestingly, all patients who survived for > 2 years after the first surgery were males < 60 years of age.
Posttransplant EPS
Three patients underwent kidney transplantation, and two of them were newly diagnosed with EPS posttransplantation. Case 11, a 38-year-old male with preserved renal function, showed the best outcome after enterolysis. In contrast, case 6 presented with severe EPS symptoms after graft failure and died of sepsis in the early phase after surgery. Case 7 with pre-existing EPS received surgical management after kidney transplantation because small bowel obstruction symptoms, including malnutrition, were aggravated and severe acute kidney injury developed. However, this patient developed enterocutaneous and internal fistulas without renal functional recovery after two successive surgeries and finally died because of sepsis.
Discussion
In this study, the overall survival rate of EPS patients was only 23.1%, and median survival durations from EPS diagnosis and the first surgery were 22 (1.3–184) and 9 (0.3–153) months, respectively. Therefore, advanced symptomatic EPS is difficult to treat by surgical management, and long-term survival (> 5 years) after surgical treatment remains poor regardless of the success of the first surgery. However, surgical intervention in the early phase of EPS may benefit young (< 60 years) male EPS patients with low CT scan scores (< 13).
Promisingly, the annual incidence of EPS is decreasing. A Dutch study reported a remarkable decrease in the annual incidence of EPS from 0.85% in 2009 to 0.14% in 2014 [22]. This decrease can be attributed to advances of PD techniques, use of physiological PD solutions (e.g., neutral solutions with low glucose degradation products), and availability of expert training to prevent peritonitis [23,24]. Nevertheless, EPS increasingly develops with prolonged PD duration. Although a high mortality rate due to irreversible EPS in patients undergoing PD has been reported with or without surgical intervention, in 2017, ISPD reported that there are insufficient data to withhold PD as the primary option of renal replacement therapy owing to the risk of EPS development.
In EPS management, developing a systemized protocol to detect and treat EPS in the early phase is important [25]. Tamoxifen with or without steroids in the early phase of EPS could be beneficial for inhibiting progression to severe EPS or recurrence [22,26].
Regarding the surgical treatment of EPS, total enterolysis relieves bowel obstruction in most EPS patients. However, enterolysis is time consuming, technically difficult, and prone to revisions and risks of various bowel complications; therefore, the surgical treatment of EPS is recommended only in patients refractory to medical treatment [27,28]. The mortality rate within 3 years after EPS diagnosis was ~50% [6,22,29], and the EPS-related mortality rate 1 year after EPS diagnosis was 42% [6]. Consistent with our results, median survival was only ~1 year after EPS diagnosis regardless of the treatment [29]. However, Kawanishi et al [19] and Ulmer et al [20] reported encouraging results with the surgical treatment of EPS in the late phase. In a Japanese study, overall mortality rates 1 and 3 years after EPS diagnosis were 7.7% and 22%, respectively, and the median survival duration after EPS diagnosis was 43.9 months [19]. In a German study, the mortality rate within 1 year after surgery was 10% [20]. Kawanishi et al [30] recently reported long-term outcomes of the surgical treatment of EPS; in that report, all-cause mortality rate was 52.7%, while the EPS-related mortality rate was 25.1% after 24 years. Kawanishi et al [28,30] performed total enterolysis with the noble plication of interintestinal suturing in order to prevent bowel kinking and re-obstruction due to adhesion, and this technique reduced the reoperation rate compared with enterolysis alone (12.3% vs. 30.4%) [19]. Ulmer et al [20] performed peritonectomy and enterolysis in 26 EPS patients to prevent recurrent EPS. Compared with simple enterolysis, peritonectomy and enterolysis together reduced the reoperation rate to 10% and improved the 1-year survival rate by decapsulation and partial deserosation. In this study, five (38.4%) patients died after the first surgery, showing poor outcomes similar to those reported in some previous studies [6,22,29] but contrary to those reported by Kawanishi et al [19] and Ulmer et al [20]. The main cause of death in these five patients was recurrent or poorly controlled infection associated with small bowel perforation or severe intra-abdominal adhesion. Relatively worse outcomes in our series compared with those in Kawanishi et al’s [30,31] study might be mainly attributed to the small study cohort and lack of cumulative experience of EPS surgical cases in our center. In contrast to the Japanese study in which postoperative perforation occurred in only eight (3.2%) patients, microperforation events requiring urgent sealing during surgery, which were related to advanced bowel degeneration and severe adhesion, frequently occurred in our series. Therefore, we encountered more frequent infection-related complications postoperatively. Accumulation of more surgical cases and experience, application of more advanced surgical techniques, such as noble plication, and development of future novel techniques for EPS management at an earlier stage are expected to improve the surgical outcomes. Nevertheless, the current high mortality rate of EPS despite surgical treatment emphasizes the importance of adequate peritonitis treatment to prevent overt EPS development.
Peritoneal thickening and calcification, bowel wall thickening and dilatation, loculated ascites, and bowel tethering due to fibrosis are common and representative CT findings in advanced EPS. Previous studies have tried to correlate these categorized CT findings in EPS cases with disease severity, such as the need for enterolysis or prolonged total parenteral nutrition and death [21,32,33]. Although Tarzi et al [21] failed to show the screening benefits of CT for EPS diagnosis, their systemized CT scoring system could be beneficial for assessing the severity and prognosis of established EPS and determining the timing of surgical intervention. Kawanishi et al [30] also reported the benefits of using a CT scoring system to assess the prognosis of EPS, although they used a modified grading system. Our results that EPS patients who survived for > 2 years achieved a low CT scan score (< 13) suggest that low CT scores in even advanced EPS cases can indicate surgical benefits.
In this study, three posttransplant EPS patients with a history of long-term PD were treated with surgical intervention; two patients were diagnosed with EPS posttransplantation and one developed EPS pretransplantation. The incidence rate of posttransplant EPS was 1% to 3% [13]. EPS usually develops in the early stage after kidney transplantation, which is supported by the two-hit hypothesis of EPS pathogenesis [8]. Similar to our results, posttransplant EPS shows better prognosis and postoperative outcomes are favorable if renal function is preserved [22,34]. Moreover, clinical resolution of established EPS has been reported after kidney transplantation, possibly due to immunosuppression [35].
In summary, this was the first study to describe the surgical management of severe EPS in a single center in Korea. Despite a high mortality rate, clinical characteristics of and prognostic factors for enterolysis in advanced EPS patients in this study may provide baseline data for further studies on more advanced surgical management. Furthermore, we recommend timely surgical intervention in young symptomatic male EPS patients with relatively low CT scores.
Supplementary Information
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Jung-Hwa Ryu, Kil-Yong Lee, and Tai Yeon Koo participated in data collection and manuscript writing. Kook-Hwan Oh, Jaeseok Yang, and Kyu Joo Park participated in study design and data analysis and interpretation. Dong Ki Kim participated in the critical review of the study design and manuscript. All authors have read and approved the final manuscript.


