| Kidney Res Clin Pract > Volume 32(2); 2013 > Article |
|
Abstract
Transplant biopsy has always been the gold standard for assessing the immune response to a kidney allograft (Chandraker A: Diagnostic techniques in the work-up of renal allograft dysfunction—an update. Curr Opin Nephrol Hypertens 8:723–728, 1999). A biopsy is not without risk and is unable to predict rejection and is only diagnostic once rejection has already occurred. However, in the past two decades, we have seen an expansion in assays that can potentially put an end to the “drug level” era, which until now has been one of the few tools available to clinicians for monitoring the immune response. A better understanding of the mechanisms of rejection and tolerance, and technological advances has led to the development of new noninvasive methods to monitor the immune response. In this article, we discuss these new methods and their potential uses in renal transplant recipients.
Keywords
Immunology, Kidney transplant, Monitoring, RecipientRecent advances in post kidney transplantation care, including development of new immunosuppressive drugs, have led to a dramatic improvement in short-term outcomes [1], [2], [3]. Yet, recipients and clinicians who have to rely on drugs that have a limited therapeutic window are caught between the rock of rejection and the hard place of side effects such as infection, toxicity, and malignancy. The inconsistent sensitivity of individuals to immunosuppressive drugs necessitates the need for assays that can measure the immune response directly and provide more information about the recipient’s immunologic status (Fig. 1). For example, in order to achieve transplantation tolerance, the Holy Grail of transplantation, it is first necessary to have a reliable and reproducible method for detecting a biomarker that is able to identify recipients in whom tolerance is likely to occur. The ideal tool for clinical monitoring should be noninvasive, inexpensive, reproducible, and accessible to clinicians and patients. Here, we summarize recent findings in biomarker identification and noninvasive immunologic monitoring based on allograft recipients' peripheral blood and urine analysis.
Methods for immunologic monitoring can broadly be divided into antigen-specific and non-antigen-specific assays. The advantage of antigen-specific assays is their ability to discriminate a donor-specific immune response from an immune response to third-party antigens.
Being antigen-specific, these assays require the knowledge of and access to donor antigens for analysis.
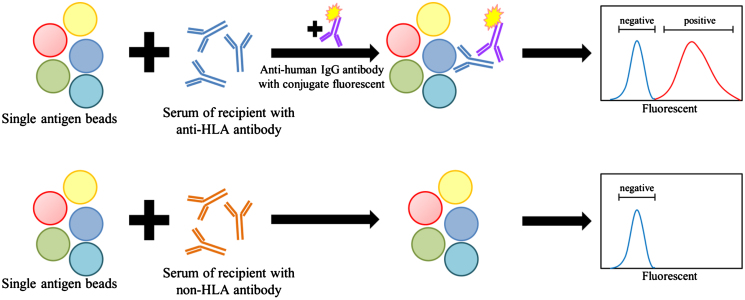
The significant impact of pretransplant donor-specific crossmatch testing on transplant outcomes is well established [4], [5]. Recent advances in solid-phase assays and flow cytometry have improved the sensitivity of the pretransplant crossmatch as well as the post-transplant monitoring of anti-HLA antibodies [6], [7]. Detection of a circulating anti-HLA antibody is now a widely used immunologic monitoring assay in the clinical setting and has been incorporated into the Banff classification system, where the presence of a donor-specific anti-HLA antibody (DSA) is considered a diagnostic criteria for antibody-mediated rejection (AMR) [8]. Microbeads with attached HLA antigens in combination with the Luminex platform are the most commonly used solid-phase assays for HLA antibody and DSA detection [9]. The tests are classified into three categories according to the variety and type of the beads in each set. The “screening set” is the simplest set, consisting of a pool of different beads of mixed HLA antigens of Class I and Class II. This set is good for general screening of anti-HLA antibody. The “single-antigen bead set” consists of single-antigen beads in groups (Fig. 2). Each bead is coated with a single HLA antigen. This is the most specific set, as it identifies the type of the anti-HLA antibody. The third category is the “panel-reactive antibody set,” which falls in between the other two sets in terms of specificity.
In the pretransplant setting, together with the complement-dependent cytotoxic crossmatch, anti-HLA antibody testing provides additional information regarding the risk of rejection, especially in highly sensitized patients [10], [11] and in the monitoring of pretransplant desensitization protocols [12], [13].
The emergence of post-transplant anti-HLA antibodies, either DSA or non-donor-specific (non-DSA), is associated with poorer kidney allograft outcomes [7], [14], [15]. DSAs have been shown to have more impact on graft outcomes than non-DSAs [14]. The presence of a post-transplantation DSA can potentially guide clinicians in the evaluation of AMR. Indeed, 40% of recipients with DSA developed AMR compared to none of the recipients with non-DSA [16]. Furthermore, the strength and breadth of the detected DSA have been correlated inconsistently with AMR risk; however, the benefit of preemptive treatment after the detection of DSA has yet to be proven.
In the mixed lymphocyte reaction, inactivated donor cells, acting as antigen-presenting cells, are mixed with recipient CD4+ T cells. The degree of T-cell proliferation is measured using radioactive thymidine or the intracellular fluorescent label carboxyfluorescein diacetate succinimidyl ester: proliferation reflects alloreactivity mainly through recognition of Class II HLA. An assay based on a similar concept is the cell-mediated lymphotoxicity assay, which measures the ability of cytotoxic T cells (CD8+) to kill donor cells: killing reflects alloreactivity via binding mainly to Class I HLA (Fig. 3).
These assays have recently been used post-transplantation, to predict the alloimmune response and guide immunosuppressive drug adjustment [17], [18]. Because of the variation in techniques and difficulty in standardizing these assays leading to discrepant results, the clinical utility of these assays in kidney transplantation is limited [19].
The enzyme-linked immunosorbent spot (ELISPOT) assay is used to detect cytokine production of alloreactive T cells. Recipient T cells are incubated with donor cells on a plate coated with an antibody specific for a certain cytokine. Donor-reactive T cells, on interaction with donor-specific cells, secrete cytokine that is captured by the antibody on the plate; once T cells are washed off the plate, a labeled second cytokine-specific antibody is added, resulting in a photoreaction where each spot represents one activated T cell. Interferon gamma (IFNγ) is the most widely used cytokine for ELISPOT. In this case, recipient T cells are incubated with donor (to detect a “direct” alloimmune response) or recipient antigen-presenting cells (to detect an indirect alloimmune response) on an anti-IFNγ-coated plate, and IFNγ-producing T cells (spots) are visualized on an immunospot image analyzer [20], [21] (Fig. 4).
Several studies have revealed a correlation between IFNγ-producing T cells detected prior to and/or after transplantation and renal transplantation outcomes [20], [22], [23], [24], [25], [26]. A higher donor-reactive T-cell response (more spots) was found in recipients who experienced acute rejection compared to recipients who had stable allograft function [20]. Bestard et al. [27] studied long-term surviving living donor kidney transplant recipients and demonstrated that circulating donor-specific alloreactive T cells are associated with graft injury and are detectable long after transplantation. Interestingly, donor-specific alloreactive T cells that correlated with allograft function and rejection were primed by the direct pathway, whereas T cells that correlated with proteinuria were primed by the indirect pathway. Another study of post-transplant donor-specific ELISPOT found that antithymocyte globulin induction prolonged donor-specific hyporesponsiveness markedly compared to IL-2 receptor blocker induction. However, both induction modalities revealed similar third-party alloreactivity [28]. Based on ELISPOT, a panel-reactive T-cell assay can be performed using a panel of allogenic stimulator cells instead of donor cells. Similar to panel-reactive antibody, pretransplant panel-reactive T-cell assay can predict post-transplant acute renal allograft rejection [29].
Recently, Heidt S, Roelen DL, de Vaal YJ, Kester MG, Eijsink C, Thomas S, van Besouw NM, Volk HD, Weimar W, Claas FH, and Mulder A [30] developed an HLA-specific B-cell ELISPOT assay using recombinant HLA monomers as the target for ELISPOT. This assay allows quantification of B cells producing specific HLA antibodies. HLA-immunized healthy individuals and patients who were on the waiting list for retransplantation revealed higher numbers of HLA-specific B cells compared to nonimmunized individuals. A large-scale study of transplant recipients is needed to validate the benefit of this new tool for monitoring humoral immunity in transplant recipients.
Post-transplant monitoring of alloreactivity by ELISPOT is promising and may be able to provide information on risk stratification, including tailoring of immunosuppressive drugs. More studies are needed to validate this concept.
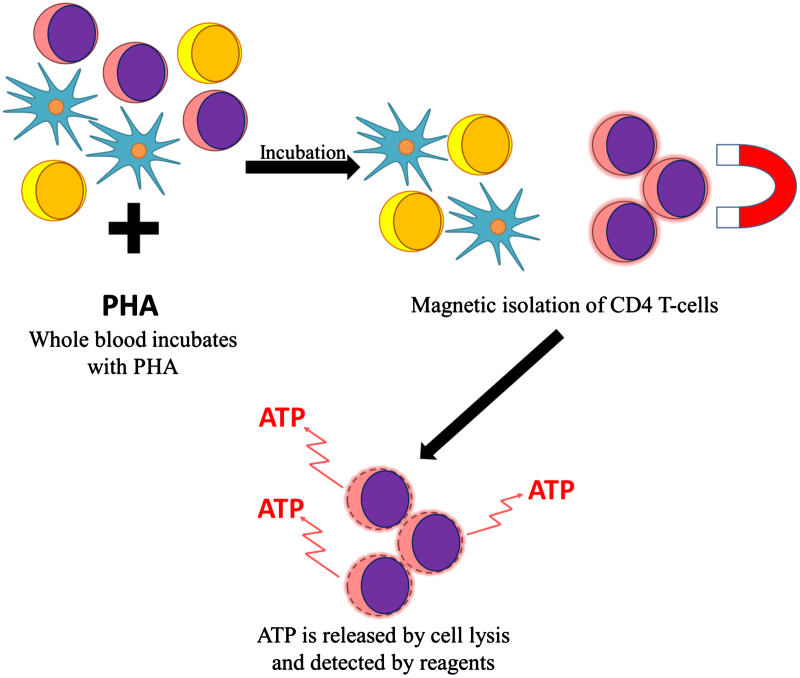
The measurement of immunosuppressive drug levels does not predict directly the reactivity of T cells. Efforts to gauge T-cell responsiveness have led to the search for assays that can measure T-cell proliferation to nonspecific stimuli. The measurement of the nucleotide adenosine triphosphate (ATP) theoretically allows a direct analysis of T-cell activity and assessment of the immunosuppressive state. The ImmuKnow assay was approved by the Food and Drug Administration (FDA) for detecting changes in activity of the immune system.
After incubating a whole blood sample, with the nonspecific mitogen phytohemagglutinin, CD4+ T cells are isolated magnetically and lysed to release ATP, which can be detected and quantified by adding a luminescent detecting reagent (Fig. 5). A higher ATP level implies a higher degree of T-cell reactivity and, therefore, potential under immunosuppression, whereas a lower level implies over immunosuppression.
A meta-analysis of 504 solid-organ transplant recipients by Kowalski et al. [31] showed that low ATP values were associated with infections, whereas high ATP values were associated with acute rejection. A prospective study of ATP release in 36 renal transplant recipients by Pérez-Flores et al. [32] showed the association between ATP values and adverse events. The recipients with a higher ATP level in the early post-transplant period were more likely to have acute rejection. The infection episodes were associated with lower ATP values. However, Huskey et al. [33] did not find any association between single time point of ATP values and acute rejection or opportunistic infections in renal transplantation recipients. Furthermore, Serban et al. [34] found that in renal transplant recipients who received thymoglobulin induction therapy, only low ATP values could predict adverse events. Higher ATP values did not predict acute rejection. Interestingly, ATP levels had no correlation with CD4+ T-cell counts but tended to correlate with total white blood cell and neutrophil counts. After phytohemagglutinin stimulation and CD4 beads isolation, 22% of cells were contaminated with myeloid cells.
Another concern is the range of ATP level, as a recent meta-analysis has revealed that within the FDA-approved predictive range of this assay (225–525 ng/mL), its sensitivity and specificity are only 0.36 and 0.80 for infection, and 0.24 and 0.73 for rejection, respectively, in renal transplant recipients [35]. By contrast, modifying the predictive range to 238–497 ng/mL was found to improve the diagnostic accuracy in a Chinese kidney transplant study [36].
Overall, although the ATP release assay is simple to perform, it is non-antigen specific and unable to diagnose infection or rejection in renal transplant recipients. Interpretation in parallel with clinical findings should be made with caution, and longitudinal monitoring seems to be more reliable than single time point measurements estimating the risk for infection or rejection.
CD30 is a member of the tumor necrosis factor receptor superfamily. In T cells, it is associated with T helper 2 (Th2) cells. CD30 has been described as a marker of memory T cells and is also found on B cells, CD8+ T cells, and natural killer (NK) cells [37], [38]. After T-cell activation, soluble CD30 (sCD30) is released into the bloodstream and can easily be detected by an enzyme-linked immunosorbent assay.
An increased level of serum sCD30 has been found in Hodgkin’s disease and immune diseases driven by Th2 cells. Some studies revealed that high pretransplant sCD30 values are associated with poor post-renal transplant outcomes [39], [40].
Prior to transplant, potential kidney recipients have higher sCD30 values compared to healthy controls [38], [41], [42]. In the early post-transplantation period, sCD30 is useful in differentiating recipients who develop acute renal allograft rejection from those with acute tubular necrosis and without complications [43]. Post-transplant measurement of sCD30 to predict late outcomes has also been studied (Table 1): higher sCD30 levels were associated with lower long-term renal allograft function and survival [40], [44], [45], [46]. Higher sCD30 levels on Day 5 and Day 7 post-transplantation were associated with later acute rejection episodes [47], [48], [49]. However, this association was not reported in other studies [50], [51].
To apply sCD30 monitoring to clinical practice, it is important to remember that sCD30 is a large molecule (120 kDa). Its values can be affected by renal function and dialysis, as it has been reported that sCD30 can be more a marker of renal function than an immunologic biomarker [42], [52], [53]. In addition, one study in healthy children showed that sCD30 concentration was affected significantly by age [54].
More studies are needed to clarify the reliability of sCD30 in immunologic monitoring, particularly early after renal transplantation, in patients with fluctuating renal function.
Flow cytometry is used for counting cells and studying the different light-scattering patterns of single cells when exposed to a light beam. This technique can widely be exploited when different cell markers are labeled with fluorescent tags.
Surface antigens, intracellular antigens, cytokines, and phosphorylated proteins can be detected using flow cytometry. Advantages of this assay not only include the small sample volume required to perform it but also the its ability to simultaneously phenotype different cell populations [55]. Because different types of lymphocytes express different markers, flow cytometry can provide information regarding the immunophenotype of each sample, such as the proportion of naïve T cells, activated CD4+ T cells, memory T cells, regulatory T cells, dendritic cells, B cells, and NK lymphocytes.
Monitoring and properly balancing the inflammatory and regulatory sides of the immune system are important in transplantation. Effector/memory T cells help prevent infection and cancer, but are associated with acute rejection [56]; they can be detected and differentiated from naïve T cells by staining for CD25, CD45RA, CD45RO, and CD62L [57], [58], [59]. Regulatory T cells, which are thought to be crucial for immunoregulation and transplant tolerance, stain positive for CD4, CD25, and FoxP3 [59], [60]. Detection of FoxP3 requires intracellular staining, fixation, and permeabilization, which impairs cell viability. Regulatory T-Cells isolation for further study and treatment using FoxP3 as a marker thus remains limited in human studies. Staining of CD127 (IL-7 receptor) provides an alternative to intracellular FoxP3 staining. In CD4+CD25+ cells, the CD127 expression was correlated inversely with FoxP3 expression, and indeed CD4+CD25+CD127lo cells showed suppressive activity [43]. Furthermore, an increased number of CD4+CD25+CD127hi-activated T cells and a decreased number of FoxP3+ regulatory T cells have been associated with chronic humoral rejection in renal transplant recipients [61], [62], whereas tolerant recipients have been found to have normal numbers of regulatory T cells similar to healthy controls.
Recently, the latency-associated peptide (LAP), the amino-terminal domain of TGF-β precursor peptide, has been identified as a novel surface marker specific for regulatory T cells [63], [64]. CD4+CD25+LAP+ T cells expressed higher levels of regulatory T-cell-associated molecules (FoxP3, glucocorticoid-induced TNFR-related gene, and CTLA-4) than CD4+CD25+LAP– cells. These CD4+CD25+LAP+ T cells also revealed suppressive function both in vitro and in vivo
[64]. This novel surface marker may possibly serve as an alternative flow cytometry detection target to intracellular FoxP3 in human studies and for potential therapeutic purposes.
Immunosuppressive drugs may have different effects on T-cell subsets. Flow cytometry can be useful in detecting such differences. Many studies have shown that calcineurin inhibitors (CNIs), but not the mammalian target of rapamycin (mTor) inhibitors, are associated with lower ratios of regulatory T cells [65], [66], [67], [68]. Recently, a modified form of CTLA4-Ig, belatacept, which blocks B7 signaling, was introduced into clinical transplantation with promising results [69]. In mice models, B7 signaling has been shown to be essential in homeostasis of regulatory T cells [70]. CTLA4-Ig treatment of human renal transplant recipients has shown similar effects on CD4+CD25+CD127lo regulatory T cells to those of CNIs [71], and their levels were comparable in pre- and post-transplant.
B cells have been recognized to play an increasingly important role in immune regulation. Depletion or deficiency of B cells has been shown, in multiple mouse models, to deteriorate immunologically mediated diseases [72]. Increased numbers of total B cells, including activated B cells, memory B cells, and early memory B cells, have been found in the peripheral blood of tolerant recipients [73]. Moreover, B cells of these recipients also expressed high levels of CD1d+ and CD5+, which are considered to be regulatory phenotypes. These findings have been confirmed by larger studies conducted by Reprogramming the Immune System for Establishment of Tolerance (RISET) and Immune Tolerance Network (ITN) [74], [75]. In addition, these studies found that tolerant recipients had increased levels of naïve (CD19+CD27-IgM+IgD+) and transitional B-cell subsets (CD19+CD24+CD38+IgD+), which also possessed immunoregulatory function. Analysis of B-cell subsets showed transitional B cells to be the most predictive population for transplant tolerance, with a sensitivity of 83% and specificity of 75% [75].
The rationale for gene expression monitoring is based on the idea that gene disruption may precede clinical or histological rejection [76]. Gene expression studies provide not only diagnostic but also predictive values [76], [77], [78]. Peripheral blood is an easy source for DNA isolation. Gene expression studies can be divided into a classic single-gene study and high-throughput microarrays, enabling the study of complete gene expression. Gene expression profiling with microarrays is increasingly used by transplant researchers for many purposes, including searching for gene profile patterns for specific conditions, identifying biomarkers for immunologic monitoring, and studying the mechanisms of rejection and tolerance [79]. However, single-gene-based testing has higher sensitivity and specificity than microarrays. A candidate gene detected by a microarray should be validated further by single-gene-based tests. Another concern in gene expression study is the transcription of gene to protein. Not all expressed genes will be transcribed to proteins. This finding may lead to discrepancies between gene profiles and clinical outcomes.
Perforin and granzyme B are secreted by CD8+ T cells and NK cells to destroy target cells, including those of the allograft, during rejection. An increased expression of perforin or granzyme B is associated with both acute cellular rejection and AMR [77], [78], [80]. Simon T, Opelz G, Wiesel M, Ott RC, and Süsal C [77] serially studied perforin and granzyme B expression from renal transplant recipients. Acute rejection could be predicted 2–30 days (median 11 days) prior to making the diagnosis of acute rejection. The expression of perforin and granzyme B mRNA in the urine of renal transplant recipients was studied by Li B, Hartono C, Ding R, Sharma VK, Ramaswamy R, Qian B, Serur D, Mouradian J, Schwartz JE, and Suthanthiran M [81]. Expression of urinary perforin and granzyme B was higher in recipients having biopsy-proven acute rejection than in those without acute rejection. Moreover, perforin exhibited a sensitivity of 83% and specificity of 83% in the prediction of acute rejection, and granzyme B showed a sensitivity and specificity of 79% and 77%, respectively. Interestingly, an increased expression of perforin and granzyme B was also found in recipients with BK virus or cytomegalovirus infection [58].
The Fas ligand is expressed by CD8+ T cells. It can induce apoptosis by binding to Fas ligands on other cells. Fas ligand mRNA expression has been also shown to be associated with acute rejection [78], [82]. However, this association has not been confirmed in all studies [83], [84].
FoxP3, the regulatory T-cell-related gene, has been also studied in the peripheral blood from renal transplant recipients. In long-term monitoring, FoxP3 mRNA levels were shown to be lower in recipients with chronic rejection than in those with stable graft function [85], [86]. Not surprisingly, high FoxP3 levels were also detected in tolerant renal transplant recipients [87]. In addition, several studies showed that FoxP3 levels increased as a protective response in acute rejection episodes; this can be applied as a diagnostic tool during acute allograft function deterioration [88], [89]. Furthermore, in a study of recipients having delayed graft function by Aquino-Dias EC, Joelsons G, da Silva DM, Berdichevski RH, Ribeiro AR, Veronese FJ, Goncalves LF, and Manfro RC [88], higher levels of FoxP3 mRNA were found in the peripheral blood of recipients with a diagnosis of acute rejection than in those with acute tubular necrosis. Another study by Muthukumar et al. [89] showed that urinary FoxP3 level was higher in recipients with acute rejection than in those with chronic allograft nephropathy and those with normal biopsy. Among recipients who experienced acute rejection, FoxP3 level was higher in those who had reversed rejection after treatment than in those who did not.
Studies have also identified biomarkers for tolerance: Toag-1 and the ratio of FoxP3 to α-1,2-mannosidase can potentially identify the tolerant recipients [90], [91]. These findings have been confirmed in a larger-scale human study [74]. Tolerant renal transplant recipients had a higher ratio of FoxP3 to α-1,2-mannosidase than those with steroid monotherapy, standard CNI treatment, and chronic allograft nephropathy.
A great advantage of this high-throughput method was recently showed in two large studies of tolerant kidney transplant recipients conducted by ITN and RISET. Many genes were found to be more expressed in tolerant recipients and, interestingly, most of them were B-cell-specific genes. Three B-cell genes, IGKV4-1, IGLL1, and IGKV1D-13, were identified by multiplex real-time polymerase chain reaction as predictors of tolerance [74], [75]. Tolerant recipients could be separated from those requiring immunosuppressive drugs and healthy controls by the expression of these B-cell-specific genes. T cells have long been recognized as an important key of allograft rejection and tolerance. Findings from these studies indicate that B cells play a role in transplantation tolerance.
Proteomics is a highly effective tool in the hunt for biomarkers. This new technique provides a high-throughput approach for studying complete sets of peptides/proteins expressed in diseases, including transplant immunology-related conditions such as acute or chronic rejection, and infection. The global study by “omics” should be followed by the classic single-molecule studies to delve into candidate peptides/proteins. Mass spectrometry is the most widely used proteomics platform.
Most of the injuries within the kidney allograft take place in the tubulointerstitium where urine formation takes place, and the paucity of background proteins in the urine, unlike serum, makes it a preferred source of sample for proteomic studies.
Acute rejection is a multistep process that starts from immune activation, includes inflammation and tubulointerstitial injury, and ends up with damage or recovery. Many proteins and peptides are expressed during this process, and can be detected by proteomic study. The candidate proteins or peptides for the ideal biomarker should be detected early in the allograft rejection process, and be able to differentiate rejection from other causes of allograft dysfunction such as tubular injury or other nonspecific causes of irreversible damage.
Schaub et al. [92] and Schaub et al. [93] reported an increase in the amount of cleaved urinary β2-microglobulin in renal allograft recipients with rejection. However, the validation study found that cleaved β2-microglobulin was unable to differentiate recipients with subclinical tubulointerstitial rejection from those with normal tubular histology [94]. Levels of β2-microglobulin were similar to those of other tubular injury markers such as neutrophil gelatinase-associated lipocalin and retinol-binding protein, in terms of tubular injury detection but not inflammation or rejection [95]. In a separate study, Schaub et al. [96] also reported that the urinary concentrations of CXCL10 and CXCL9, the CXC-receptor 3 (CXCR3) proinflammatory chemokines, were significantly higher in subclinical tubulitis than in subclinical borderline tubulitis and normal histology.
O’Riordan et al. [97] and O’Riordan et al. [98] have identified β-defensin-1 (a 4.7 kDa peptide) and α-1-antichymotrypsin (a 4.4 kDa peptide) as useful markers in diagnosing acute renal allograft rejection, as both are involved in the inflammatory process. Sigdel et al. [99] found that urine of recipients with allograft rejection have lower levels of Tamm–Horsfall protein (uromodulin) and CD44, and higher levels of pigment epithelium-derived factor compared to that of stable allograft function recipients and healthy controls. Because this study did not enroll recipients with other causes of renal allograft dysfunction, we are unable to conclude that the alteration of these proteins was solely caused by the rejection process. In some studies, other distinct peptides have also been identified in the urine of renal allograft rejection recipients [100], [101], [102]. However, a definite protein or biomarker has yet to be identified.
Addition and deletion of nucleotides during the generation of a T-cell receptor (TCR) by rearrangement occur in a random manner. The complementary-determining region 3 (CDR3) of Vβ gene of the TCR is also created randomly and leads to diversity in the length of the CDR3. This random rearrangement process leads to a Gaussian distribution of CDR3 length. In transplant recipients, a deviation from the Gaussian distribution of CDR3 length indicates clonal expansion of T-cell population, which reflects T-cell activation or rejection [103].
Miqueu et al. [104] reported a large, multicenter, case-controlled study of TCR repertoire by polymerase chain reaction in peripheral blood of renal transplant recipients using “Tc Landscape,” a statistical method developed for analyzing the TCR repertoire distribution. Of the tolerant recipients, 92.8% were found to have a normal distribution pattern or mild kurtosis pattern compared to 42.8% of recipients with biopsy-proven chronic humoral rejection. This demonstrates the association between TCR repertoire and allogenic immunity.
In conclusion, because manipulation of the immune system is key to transplantation, monitoring of the immunological response is crucial in understanding the environment in which the allograft functions in any given individual. Currently, there is no best immunological monitoring method, but promising advancements have been achieved over the past few years. With the development of these technologies, understanding the strengths and weaknesses of each test will allow clinicians to integrate these monitoring methods with clinical assessment to achieve the best long-term outcomes in transplant recipients.
References
1. Chandraker A.. Diagnostic techniques in the work-up of renal allograft dysfunction—an update. Curr Opin Nephrol Hypertens 8:1999;723–728.


2. Kasiske B.L., Gaston R.S., Gourishankar S., Halloran P.F., Matas A.J., Jeffery J., Rush D.. Long-term deterioration of kidney allograft function. Am J Transplant 5:2005;1405–1414.


3. Sayegh M.H., Carpenter C.B.. Transplantation 50 years later—progress, challenges, and promises. N Engl J Med 351:2004;2761–2766.


4. Terasaki P.I., Kreisler M., Mickey R.M.. Presensitization and kidney transplant failures. Postgrad Med J 47:1971;89–100.



5. Patel R., Terasaki P.I.. Significance of the positive crossmatch test in kidney transplantation. N Engl J Med 280:1969;735–739.


6. Pei R., Lee J.H., Shih N.J., Chen M., Terasaki P.I.. Single human leukocyte antigen flow cytometry beads for accurate identification of human leukocyte antigen antibody specificities. Transplantation 75:2003;43–49.


7. Li X., Ishida H., Yamaguchi Y., Tanabe K.. Poor graft outcome in recipients with de novo donor-specific anti-HLA antibodies after living related kidney transplantation. Transpl Int 21:2008;1145–1152.


8. Sis B., Mengel M., Haas M., Colvin R.B., Halloran P.F., Racusen L.C., Solez K., Baldwin W.M. 3rd, Bracamonte E.R., Broecker V., Cosio F., Demetris A.J., Drachenberg C., Einecke G., Gloor J., Glotz D., Kraus E., Legendre C., Liapis H., Mannon R.B., Nankivell B.J., Nickeleit V., Papadimitriou J.C., Randhawa P., Regele H., Renaudin K., Rodriguez E.R., Seron D., Seshan S., Suthanthiran M., Wasowska B.A., Zachary A., Zeevi A.. Banff '09 meeting report: antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10:2010;464–471.


9. Cecka J.M.. Current methodologies for detecting sensitization to HLA antigens. Curr Opin Organ Transplant 16:2011;398–403.


10. Mulley W.R., Kanellis J.. Understanding crossmatch testing in organ transplantation: a case-based guide for the general nephrologist. Nephrology (Carlton) 16:2011;125–133.


11. Patel A.M., Pancoska C., Mulgaonkar S., Weng F.L.. Renal transplantation in patients with pre-transplant donor-specific antibodies and negative flow cytometry crossmatches. Am J Transplant 7:2007;2371–2377.


12. Montgomery R.A., Lonze B.E., King K.E., Kraus E.S., Kucirka L.M., Locke J.E., Warren D.S., Simpkins C.E., Dagher N.N., Singer A.L., Zachary A.A., Segev D.L.. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med 365:2011;318–326.


13. Marfo K., Lu A., Ling M., Akalin E.. Desensitization protocols and their outcome. Clin J Am Soc Nephrol 6:2011;922–936.


14. Ntokou I.S., Iniotaki A.G., Kontou E.N., Darema M.N., Apostolaki M.D., Kostakis A.G., Boletis J.N.. Long-term follow up for anti-HLA donor specific antibodies postrenal transplantation: high immunogenicity of HLA class II graft molecules. Transpl Int 24:2011;1084–1093.


15. Terasaki P.I., Ozawa M., Castro R.. Four-year follow-up of a prospective trial of HLA and MICA antibodies on kidney graft survival. Am J Transplant 7:2007;408–415.


16. Ishida H., Hirai T., Kohei N., Yamaguchi Y., Tanabe K.. Significance of qualitative and quantitative evaluations of anti-HLA antibodies in kidney transplantation. Transpl Int 24:2011;150–157.


17. Weimar W., Rischen-Vos J., de Kuiper P., Gregoor P.J., JN I.J., van Besouw N.M., Baan C.C., van der Mast B.J.. Tapering immunosuppression in recipients of living donor kidney transplants. Nephrol Dial Transplant 19(Suppl 4):2004;iv61–iv63.


18. van Besouw N.M., van der Mast B.J., de Kuiper P., Smak Gregoor P.J., Vaessen L.M., IJzermans J.N., van Gelder T., Weimar W.. Donor-specific T-cell reactivity identifies kidney transplant patients in whom immunosuppressive therapy can be safely reduced. Transplantation 70:2000;136–143.

19. Reinsmoen N.L.. Cellular methods used to evaluate the immune response in transplantation. Tissue antigens 59:2002;241–250.


20. Gebauer B.S., Hricik D.E., Atallah A., Bryan K., Riley J., Tary-Lehmann M., Greenspan N.S., Dejelo C., Boehm B.O., Hering B.J., Heeger P.S.. Evolution of the enzyme-linked immunosorbent spot assay for post-transplant alloreactivity as a potentially useful immune monitoring tool. Am J Transplant 2:2002;857–866.


21. Babel N., Reinke P., Volk H.D.. Lymphocyte markers and prediction of long-term renal allograft acceptance. Curr Opin Nephrol Hypertens 18:2009;489–494.


22. Nather B.J., Nickel P., Bold G., Presber F., Schonemann C., Pratschke J., Volk H.D., Reinke P.. Modified ELISPOT technique—highly significant inverse correlation of post-Tx donor-reactive IFN gamma-producing cell frequencies with 6 and 12 months graft function in kidney transplant recipients. Transpl Immunol 16:2006;232–237.

23. Nickel P., Presber F., Bold G., Biti D., Schonemann C., Tullius S.G., Volk H.D., Reinke P.. Enzyme-linked immunosorbent spot assay for donor-reactive interferon-gamma-producing cells identifies T-cell presensitization and correlates with graft function at 6 and 12 months in renal-transplant recipients. Transplantation 78:2004;1640–1646.


24. Najafian N., Salama A.D., Fedoseyeva E.V., Benichou G., Sayegh M.H.. Enzyme-linked immunosorbent spot assay analysis of peripheral blood lymphocyte reactivity to donor HLA-DR peptides: potential novel assay for prediction of outcomes for renal transplant recipients. J Am Soc Nephrol 13:2002;252–259.


25. Kim S.H., Oh E.J., Kim M.J., Park Y.J., Han K., Yang H.J., Kim J.Y., Choi B.S., Yang C.W., Kim Y.S., Bang B.K.. Pretransplant donor-specific interferon-gamma ELISPOT assay predicts acute rejection episodes in renal transplant recipients. Transplant Proc 39:2007;3057–3060.


26. Reinsmoen N.L., Cornett K.M., Kloehn R., Burnette A.D., McHugh L., Flewellen B.K., Matas A., Savik K.. Pretransplant donor-specific and non-specific immune parameters associated with early acute rejection. Transplantation 85:2008;462–470.


27. Bestard O., Nickel P., Cruzado J.M., Schoenemann C., Boenisch O., Sefrin A., Grinyo J.M., Volk H.D., Reinke P.. Circulating alloreactive T cells correlate with graft function in longstanding renal transplant recipients. J Am Soc Nephrol 19:2008;1419–1429.



28. Cherkassky L., Lanning M., Lalli P.N., Czerr J., Siegel H., Danziger-Isakov L., Srinivas T., Valujskikh A., Shoskes D.A., Baldwin W., Fairchild R.L., Poggio E.D.. Evaluation of alloreactivity in kidney transplant recipients treated with antithymocyte globulin versus IL-2 receptor blocker. Am J Transplant 11:2011;1388–1396.



29. Poggio E.D., Augustine J.J., Clemente M., Danzig J.M., Volokh N., Zand M.S., Hricik D.E., Heeger P.S.. Pretransplant cellular alloimmunity as assessed by a panel of reactive T cells assay correlates with acute renal graft rejection. Transplantation 83:2007;847–852.


30. Heidt S., Roelen D.L., de Vaal Y.J., Kester M.G., Eijsink C., Thomas S., van Besouw N.M., Volk H.D., Weimar W., Claas F.H., Mulder A.. A novel ELISPOT assay to quantify HLA-specific B cells in HLA-immunized individuals. Am J Transplant 12:2012;1469–1478.


31. Kowalski R.J., Post D.R., Mannon R.B., Sebastian A., Wright H.I., Sigle G., Burdick J., Elmagd K.A., Zeevi A., López-Cepero M., Daller J.A., Gritsch H.A., Reed E.F., Jonsson J., Hawkins D., Britz J.A.. Assessing relative risks of infection and rejection: a meta-analysis using an immune function assay. Transplantation 82:2006;663–668.


32. Perez-Flores I., Sanchez-Fructuoso A., Santiago J.L., Fernandez-Arquero M., Calvo N., de la Concha E.G., Barrientos A.. Intracellular ATP levels in CD4+ lymphocytes are a risk marker of rejection and infection in renal graft recipients. Transplant Proc 41:2009;2106–2108.


33. Huskey J., Gralla J., Wiseman A.C.. Single time point immune function assay (ImmuKnow) testing does not aid in the prediction of future opportunistic infections or acute rejection. Clin J Am Soc Nephrol 6:2011;423–429.



34. Serban G., Whittaker V., Fan J., Liu Z., Manga K., Khan M., Kontogianni K., Padmanabhan A., Cohen D., Suciu-Foca N., Ratner L., Colovai A.I.. Significance of immune cell function monitoring in renal transplantation after Thymoglobulin induction therapy. Hum Immunol 70:2009;882–890.


35. Ling X., Xiong J., Liang W., Schroder P.M., Wu L., Ju W., Kong Y., Shang Y., Guo Z., He X.. Can immune cell function assay identify patients at risk of infection or rejection? A meta-analysis. Transplantation 93:2012;737–743.


36. Zhou H., Wu Z., Ma L., Wu W., Yang S., Wang Q., Yuan X., Wu L., Lin X., Tan J.. Assessing immunologic function through CD4 T-lymphocyte ahenosine triphosphate levels by ImmuKnow assay in Chinese patients following renal transplantation. Transplant Proc 43:2011;2574–2578.


37. Kennedy M.K., Willis C.R., Armitage R.J.. Deciphering CD30 ligand biology and its role in humoral immunity. Immunology 118:2006;143–152.



38. Altermann W., Schlaf G., Rothhoff A., Seliger B.. High variation of individual soluble serum CD30 levels of pre-transplantation patients: sCD30 a feasible marker for prediction of kidney allograft rejection? Nephrol Dial Transplant 22:2007;2795–2799.


39. Cinti P., Pretagostini R., Arpino A., Tamburro M.L., Mengasini S., Lattanzi R., De Simone P., Berloco P., Molajoni E.R.. Evaluation of pretransplant immunologic status in kidney-transplant recipients by panel reactive antibody and soluble CD30 determinations. Transplantation 79:2005;1154–1156.


40. Süsal C., Pelzl S., Opelz G.. Strong human leukocyte antigen matching effect in nonsensitized kidney recipients with high pretransplant soluble CD30. Transplantation 76:2003;1231–1232.


41. Sengul S., Keven K., Gormez U., Kutlay S., Erturk S., Erbay B.. Identification of patients at risk of acute rejection by pretransplantation and posttransplantation monitoring of soluble CD30 levels in kidney transplantation. Transplantation 81:2006;1216–1219.


42. Barbano G., Cappa F., Prigione I., Pistoia V., Cohen A., Chiesa S., Gusmano R., Perfumo F.. Plasma levels of soluble CD30 are increased in children with chronic renal failure and with primary growth deficiency and decrease during treatment with recombination human growth hormone. Nephrol Dial Transplant 16:2001;1807–1813.


43. Pelzl S., Opelz G., Daniel V., Wiesel M., Süsal C.. Evaluation of posttransplantation soluble CD30 for diagnosis of acute renal allograft rejection. Transplantation 75:2003;421–423.


44. Weimer R., Süsal C., Yildiz S., Staak A., Pelzl S., Renner F., Dietrich H., Daniel V., Kamali-Ernst S., Ernst W., Padberg W., Opelz G.. Post-transplant sCD30 and neopterin as predictors of chronic allograft nephropathy: impact of different immunosuppressive regimens. Am J Transplant 6:2006;1865–1874.


45. Hamer R., Roche L., Smillie D., Harmer A., Mitchell D., Molostvov G., Lam F.T., Kashi H., Tan L.C., Imray C., Fletcher S., Briggs D., Lowe D., Zehnder D., Higgins R.. Soluble CD30 and Cd27 levels in patients undergoing HLA antibody-incompatible renal transplantation. Transpl Immunol 23:2010;161–165.


46. Langan L.L., D'Orsogna L., Park L.P., Hughes T.L., Irish A., Luxton G., Witt C.S., Christiansen F.T.. HLA antibodies and soluble CD30 are associated with poor renal graft outcome: updated results of a single-center cross-sectional study. Clin Transpl 2006;219–225.

47. Yang J.L., Hao H.J., Zhang B., Liu Y.X., Chen S., Na Y.Q.. Level of soluble CD30 after kidney transplantation correlates with acute rejection episodes. Transplant Proc 40:2008;3381–3383.


48. Domingues E.M., Matuck T., Graciano M.L., Souza E., Rioja S., Falci M.C.. Monteiro de Carvalho DB, Porto LC: Panel reactive HLA antibodies, soluble CD30 levels, and acute rejection six months following renal transplant. Clin Transplant 24:2010;821–829.


49. Wang D., Wu G.J., Wu W.Z., Yang S.L., Chen J.H., Wang H., Lin W.H., Wang Q.H., Zeng Z.X., Tan J.M.. Pre- and post-transplant monitoring of soluble CD30 levels as predictor of acute renal allograft rejection. Transpl Immunol 17:2007;278–282.


50. Slavcev A., Honsova E., Lodererova A., Pavlova Y., Sajdlova H., Vitko S., Skibova J., Striz I., Viklicky O.. Soluble CD30 in patients with antibody-mediated rejection of the kidney allograft. TransplImmunol 18:2007;22–27.

51. Platt R.E., Wu K.S., Poole K., Newstead C.G., Clark B.. Soluble CD30 as a prognostic factor for outcome following renal transplantation. J Clin Pathol 62:2009;662–663.


52. Lopez-Hoyos M., San Segundo D., Benito M.J., Fernandez-Fresnedo G., Ruiz J.C., Rodrigo E., Gomez-Alamillo C., Benito A., Arias M.. Association between serum soluble CD30 and serum creatinine before and after renal transplantation. Transplant Proc 40:2008;2903–2905.


53. Nakao K., Nagake Y., Okamoto A., Ichikawa H., Yamamura M., Makino H.. Serum levels of soluble CD26 and CD30 in patients on hemodialysis. Nephron 91:2002;215–221.


54. Chrul S., Polakowska E.. Age-dependent changes of serum soluble CD30 concentration in children. Pediatr Transplant 15:2011;515–518.


55. Dieterlen M.T., Eberhardt K., Tarnok A., Bittner H.B., Barten M.J.. Flow cytometry-based pharmacodynamic monitoring after organ transplantation. Methods Cell Biol 103:2011;267–284.


56. Pearl J.P., Parris J., Hale D.A., Hoffmann S.C., Bernstein W.B., McCoy K.L., Swanson S.J., Mannon R.B., Roederer M., Kirk A.D.. Immunocompetent T-cells with a memory-like phenotype are the dominant cell type following antibody-mediated T-cell depletion. Am J Transplant 5:2005;465–474.


57. Seddiki N., Santner-Nanan B., Martinson J., Zaunders J., Sasson S., Landay A., Solomon M., Selby W., Alexander S.I., Nanan R., Kelleher A.. Fazekas de St Groth B: Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med 203:2006;1693–1700.



58. Heidt S., San Segundo D., Shankar S., Mittal S., Muthusamy A.S., Friend P.J., Fuggle S.V., Wood K.J.. Peripheral blood sampling for the detection of allograft rejection: biomarker identification and validation. Transplantation 92:2011;1–9.


59. Sakaguchi S., Miyara M., Costantino C.M., Hafler D.A.. FOXP3+ regulatory T cells in the human immune system. Nat Rev Immunol 10:2010;490–500.


60. Wood K.J., Sakaguchi S.. Regulatory T cells in transplantation tolerance. Nat Rev Immunol 3:2003;199–210.


61. Vallotton L., Hadaya K., Venetz J.P., Buehler L.H., Ciuffreda D., Nseir G., Codarri L., Villard J., Pantaleo G., Pascual M.. Monitoring of CD4+CD25highIL-7Ralphahigh activated T cells in kidney transplant recipients. Clin J Am Soc Nephrol 6:2011;2025–2033.



62. Braudeau C., Racape M., Giral M., Louis S., Moreau A., Berthelot L., Heslan M., Ashton-Chess J., Soulillou J.P., Brouard S.. Variation in numbers of CD4+CD25highFOXP3+ T cells with normal immuno-regulatory properties in long-term graft outcome. Transpl Int 20:2007;845–855.


63. Tran D.Q., Andersson J., Hardwick D., Bebris L., Illei G.G., Shevach E.M.. Selective expression of latency-associated peptide (LAP) and IL-1 receptor type I/II (CD121a/CD121b) on activated human FOXP3+ regulatory T cells allows for their purification from expansion cultures. Blood 113:2009;5125–5133.



64. Chen M.L., Yan B.S., Bando Y., Kuchroo V.K., Weiner H.L.. Latency-associated peptide identifies a novel CD4+CD25+ regulatory T cell subset with TGFbeta-mediated function and enhanced suppression of experimental autoimmune encephalomyelitis. J Immunol 180:2008;7327–7337.



65. San Segundo D., Fabrega E.. Reduced numbers of blood natural regulatory T cells in stable liver transplant recipients with high levels of calcineurin inhibitors. Transplant Proc 39:2007;2290–2292.


66. Segundo D.S., Ruiz J.C., Izquierdo M., Fernandez-Fresnedo G., Gomez-Alamillo C., Merino R., Benito M.J., Cacho E., Rodrigo E., Palomar R., Lopez-Hoyos M., Arias M.. Calcineurin inhibitors, but not rapamycin, reduce percentages of CD4+CD25+FOXP3+ regulatory T cells in renal transplant recipients. Transplantation 82:2006;550–557.


67. Demirkiran A., Kok A., Kwekkeboom J., Metselaar H.J., Tilanus H.W., van der Laan L.J.. Decrease of CD4+CD25+ T cells in peripheral blood after liver transplantation: association with immunosuppression. Transplant Proc 37:2005;1194–1196.


68. Hendrikx T.K., Velthuis J.H., Klepper M., van Gurp E., Geel A., Schoordijk W., Baan C.C., Weimar W.. Monotherapy rapamycin allows an increase of CD4 CD25 FoxP3 T cells in renal recipients. Transpl Int 22:2009;884–891.


69. Vincenti F., Blancho G., Durrbach A., Friend P., Grinyo J., Halloran P.F., Klempnauer J., Lang P., Larsen C.P., Muhlbacher F., Nashan B., Soulillou J.P., Vanrenterghem Y., Wekerle T., Agarwal M., Gujrathi S., Shen J., Shi R., Townsend R., Charpentier B.. Five-year safety and efficacy of belatacept in renal transplantation. J Am Soc Nephrol 21:2010;1587–1596.



70. Salomon B., Lenschow D.J., Rhee L., Ashourian N., Singh B., Sharpe A., Bluestone J.A.. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12:2000;431–440.


71. Bluestone J.A., Liu W., Yabu J.M., Laszik Z.G., Putnam A., Belingheri M., Gross D.M., Townsend R.M., Vincenti F.. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant 8:2008;2086–2096.



72. Ashoor I.F., Najafian N.. Rejection and regulation: a tight balance. Curr Opin Organ Transplant 17:2012;1–7.


73. Pallier A., Hillion S., Danger R., Giral M., Racape M., Degauque N., Dugast E., Ashton-Chess J., Pettre S., Lozano J.J., Bataille R., Devys A., Cesbron-Gautier A., Braudeau C., Larrose C., Soulillou J.P., Brouard S.. Patients with drug-free long-term graft function display increased numbers of peripheral B cells with a memory and inhibitory phenotype. Kidney Int 78:2010;503–513.


74. Sagoo P., Perucha E., Sawitzki B., Tomiuk S., Stephens D.A., Miqueu P., Chapman S., Craciun L., Sergeant R., Brouard S., Rovis F., Jimenez E., Ballow A., Giral M., Rebollo-Mesa I., Le Moine A., Braudeau C., Hilton R., Gerstmayer B., Bourcier K., Sharif A., Krajewska M., Lord G.M., Roberts I., Goldman M., Wood K.J., Newell K., Seyfert-Margolis V., Warrens A.N., Janssen U., Volk H.D., Soulillou J.P., Hernandez-Fuentes M.P., Lechler R.I.. Development of a cross-platform biomarker signature to detect renal transplant tolerance in humans. J Clin Invest 120:2010;1848–1861.



75. Newell K.A., Asare A., Kirk A.D., Gisler T.D., Bourcier K., Suthanthiran M., Burlingham W.J., Marks W.H., Sanz I., Lechler R.I., Hernandez-Fuentes M.P., Turka L.A., Seyfert-Margolis V.L.. Identification of a B cell signature associated with renal transplant tolerance in humans. J Clin Invest 120:2010;1836–1847.



76. Anglicheau D., Suthanthiran M.. Noninvasive prediction of organ graft rejection and outcome using gene expression patterns. Transplantation 86:2008;192–199.



77. Simon T., Opelz G., Wiesel M., Ott R.C., Süsal C.. Serial peripheral blood perforin and granzyme B gene expression measurements for prediction of acute rejection in kidney graft recipients. Am J Transplant 3:2003;1121–1127.


78. Vasconcellos L.M., Schachter A.D., Zheng X.X., Vasconcellos L.H., Shapiro M., Harmon W.E., Strom T.B.. Cytotoxic lymphocyte gene expression in peripheral blood leukocytes correlates with rejecting renal allografts. Transplantation 66:1998;562–566.


79. Zarkhin V., Sarwal M.M.. Microarrays: monitoring for transplant tolerance and mechanistic insights. Clin Lab Med 28:2008 385–410. vi.


80. Veale J.L., Liang L.W., Zhang Q., Gjertson D.W., Du Z., Bloomquist E.W., Jia J., Qian L., Wilkinson A.H., Danovitch G.M., Pham P.T., Rosenthal J.T., Lassman C.R., Braun J., Reed E.F., Gritsch H.A.. Noninvasive diagnosis of cellular and antibody-mediated rejection by perforin and granzyme B in renal allografts. Hum Immunol 67:2006;777–786.


81. Li B., Hartono C., Ding R., Sharma V.K., Ramaswamy R., Qian B., Serur D., Mouradian J., Schwartz J.E., Suthanthiran M.. Noninvasive diagnosis of renal-allograft rejection by measurement of messenger RNA for perforin and granzyme B in urine. N Engl J Med 344:2001;947–954.


82. Netto M.V., Fonseca B.A., Dantas M.. Saber LT, Castro MC, Ferraz AS: Granzyme B, FAS-ligand and perforin expression during acute cellular rejection episodes after kidney transplantation: comparison between blood and renal aspirates. Transplant Proc 34:2002;476–478.


83. Shin G.T., Kim S.J., Lee T.S., Oh C.K., Kim H.. Gene expression of perforin by peripheral blood lymphocytes as a marker of acute rejection. Nephron Clin Pract 100:2005;c63–c70.


84. Graziotto R., Del Prete D., Rigotti P., Anglani F., Baldan N., Furian L., Valente M., Antonello A., Marchini F., D'Angelo A., Gambaro G.. Perforin, Granzyme B, and fas ligand for molecular diagnosis of acute renal-allograft rejection: analyses on serial biopsies suggest methodological issues. Transplantation 81:2006;1125–1132.


85. Iwase H., Kobayashi T., Kodera Y., Miwa Y., Kuzuya T., Iwasaki K., Haneda M., Katayama A., Takeda A., Morozumi K., Watarai Y., Uchida K., Nakao A.. Clinical significance of regulatory T-cell-related gene expression in peripheral blood after renal transplantation. Transplantation 91:2011;191–198.


86. Alvarez C.M., Opelz G., Garcia L.F., Süsal C.. Expression of regulatory T-cell-related molecule genes and clinical outcome in kidney transplant recipients. Transplantation 87:2009;857–863.


87. Moraes-Vieira P.M., Takenaka M.C., Silva H.M., Monteiro S.M., Agena F., Lemos F., Saitovitch D., Kalil J., Coelho V.. GATA3 and a dominant regulatory gene expression profile discriminate operational tolerance in human transplantation. Clin Immunol 142:2012;117–126.


88. Aquino-Dias E.C., Joelsons G., da Silva D.M., Berdichevski R.H., Ribeiro A.R., Veronese F.J., Goncalves L.F., Manfro R.C.. Non-invasive diagnosis of acute rejection in kidney transplants with delayed graft function. Kidney Int 73:2008;877–884.


89. Muthukumar T., Dadhania D., Ding R., Snopkowski C., Naqvi R., Lee J.B., Hartono C., Li B., Sharma V.K., Seshan S.V., Kapur S., Hancock W.W., Schwartz J.E., Suthanthiran M., Messenger R.N.A.. for FOXP3 in the urine of renal-allograft recipients. N Engl J Med 353:2005;2342–2351.


90. Sawitzki B., Schlickeiser S., Reinke P., Volk H.D.. Monitoring tolerance and rejection in organ transplant recipients. Biomarkers 16(Suppl 1):2011;S42–S50.


91. Sawitzki B., Bushell A., Steger U., Jones N., Risch K., Siepert A., Lehmann M., Schmitt-Knosalla I., Vogt K., Gebuhr I., Wood K., Volk H.D.. Identification of gene markers for the prediction of allograft rejection or permanent acceptance. Am J Transplant 7:2007;1091–1102.


92. Schaub S., Rush D., Wilkins J., Gibson I.W., Weiler T., Sangster K., Nicolle L., Karpinski M., Jeffery J., Nickerson P.. Proteomic-based detection of urine proteins associated with acute renal allograft rejection. J Am Soc Nephrol 15:2004;219–227.


93. Schaub S., Wilkins J.A., Antonovici M., Krokhin O., Weiler T., Rush D., Nickerson P.. Proteomic-based identification of cleaved urinary beta2-microglobulin as a potential marker for acute tubular injury in renal allografts. Am J Transplant 5:2005;729–738.


94. Schaub S., Mayr M., Honger G., Bestland J., Steiger J., Regeniter A., Mihatsch M.J., Wilkins J.A., Rush D., Nickerson P.. Detection of subclinical tubular injury after renal transplantation: comparison of urine protein analysis with allograft histopathology. Transplantation 84:2007;104–112.


95. Schaub S., Wilkins J.A., Nickerson P.. Proteomics and renal transplantation: searching for novel biomarkers and therapeutic targets. Contrib Nephrol 160:2008;65–75.


96. Schaub S., Nickerson P., Rush D., Mayr M., Hess C., Golian M., Stefura W., Hayglass K.. Urinary CXCL9 and CXCL10 levels correlate with the extent of subclinical tubulitis. Am J Transplant 9:2009;1347–1353.


97. O'Riordan E., Orlova T.N., Mei J.J., Butt K., Chander P.M., Rahman S., Mya M., Hu R., Momin J., Eng E.W., Hampel D.J., Hartman B., Kretzler M., Delaney V., Goligorsky M.S.. Bioinformatic analysis of the urine proteome of acute allograft rejection. J Am Soc Nephrol 15:2004;3240–3248.


98. O'Riordan E., Orlova T.N., Podust V.N., Chander P.N., Yanagi S., Nakazato M., Hu R., Butt K., Delaney V., Goligorsky M.S.. Characterization of urinary peptide biomarkers of acute rejection in renal allografts. Am J Transplant 7:2007;930–940.


99. Sigdel T.K., Kaushal A., Gritsenko M., Norbeck A.D., Qian W.J., Xiao W., Camp D.G. 2nd, Smith R.D., Sarwal M.M.. Shotgun proteomics identifies proteins specific for acute renal transplant rejection. Proteomics Clin Appl 4:2010;32–47.




100. Ann. Surg.
102. Wittke S., Haubitz M., Walden M., Rohde F., Schwarz A., Mengel M., Mischak H., Haller H., Gwinner W.. Detection of acute tubulointerstitial rejection by proteomic analysis of urinary samples in renal transplant recipients. Am J Transplant 5:2005;2479–2488.


103. Naesens M., Sarwal M.M.. Harnessing the diversity of the human T-cell repertoire: a monitoring tool for transplantation tolerance? Eur J Immunol 40:2010;2986–2989.


104. Miqueu P., Degauque N., Guillet M., Giral M., Ruiz C., Pallier A., Braudeau C., Roussey-Kesler G., Ashton-Chess J., Dore J.C., Thervet E., Legendre C., Hernandez-Fuentes M.P., Warrens A.N., Goldman M., Volk H.D., Janssen U., Wood K.J., Lechler R.I., Bertrand D., Sebille V., Soulillou J.P., Brouard S.. Analysis of the peripheral T-cell repertoire in kidney transplant patients. Eur J Immunol 40:2010;3280–3290.


Figure 3
Mixed lymphocyte reaction and cell-mediated lymphocytotoxicity. APC, antigen-presenting cell.

Table 1
Studies of sCD30 in peripheral blood of post renal transplant recipients
| References | Outcome measured | Results |
|---|---|---|
| Weimer et al. [44] | Allograft function | Higher sCD30 level at 1 y associated with lower allograft function at 2 y |
| CMV disease associated with transient elevation of sCD30 level | ||
| Langan et al. [46] | Allograft survival | Higher sCD30 level in 1 y associated with poorer 6-y allograft survival |
| Wang et al. [49] | Acute rejection | Higher sCD30 level at Day 5 associated with acute rejection |
| Slavcev et al. [50] | Acute humoral rejection | sCD30 level not associated with acute humoral rejection |
| Yang et al. [47] | Acute rejection | Higher sCD30 level at Day 7 associated with acute rejection |
| Lopez-Hoyos et al. [52] | Allograft function | Significant correlation between sCD30 and serum creatinine at all times of the study |
| Hamer et al. [45] | Acute rejection | Higher sCD30 level at 4–6 wks not associated with acute rejection, but associated with poorer allograft function at 1 y |
| Allograft function | ||
| Domingues et al. [48] | Acute rejection | Higher sCD30 level at Day 7 associated with acute rejection |
| Süsal et al. [40] | Allograft survival | Higher sCD30 level at Day 30 associated with poorer 3-y allograft survival |
- TOOLS
-
METRICS

- Related articles
-
Necessity of induction agent modification for old age kidney transplant recipients
Rhabdomyolysis Induced by Hypothyroidism in a Kidney Transplant Recipient2011 January;30(1)
Cerebral Aspergillosis in a Kidney Transplant Patient2010 March;29(2)
Antiviral Therapy Hepatitis B in Renal Transplant Recipients 2006 November;25(6)





 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print