Clinical outcomes in pediatric patients with normal renal histopathology
Article information
Abstract
Background
There have been some cases where abnormal histopathologic findings could not be found in the kidney could even with proper specimen collection through percutaneous renal biopsy (PRB) in accordance with its indication. We analyzed the incidence and clinical outcomes of children who showed normal histopathological findings in their PRBs.
Methods
The medical records of 552 pediatric subjects who underwent PRB between 2005 and 2016 were reviewed. Twenty-six subjects were excluded because allograft biopsy was performed in nine subjects, and the age at biopsy was greater than 18 years in 17 subjects. Finally, 526 subjects were enrolled in this study.
Results
Of the 526 pediatric patients, 32 (6.1%) showed no histopathological abnormalities in their PRBs. The male-to-female ratio of the patients was 1.9:1, and the mean ages at the first visit and at biopsy were 10.6 ± 4.1 and 11.4 ± 3.8 years, respectively. In accordance with the biopsy indications, recurrent gross hematuria showed the highest incidence rate, but combined hematuria and proteinuria had the lowest incidence rate regarding normal renal histopathology among all the subjects. At a mean follow-up of 35.5 ± 23.6 months, urinary abnormalities had improved in more than 50% of the subjects with normal renal histopathology, and none of the patients showed progression to end-stage renal disease or required rebiopsy due to symptom worsening during the follow-up period.
Conclusion
The clinical outcomes of children with normal PRB histopathologic findings are generally good. Further studies to evaluate their long-term outcomes are needed.
Introduction
Percutaneous renal biopsy (PRB) is an important and valuable procedure for the diagnosis of renal diseases. It provides valuable information to determine a treatment plan and predict prognosis. PRB using an aspiration biopsy needle was first introduced in 1951 by Iversen and Brun [1]. Kark and Muehrcke [2] introduced a technique using a Vim-Silverman needle in the prone position, greatly improving the success rate of renal biopsy. At present, ultrasonography-guided methods and automated guns have been widely used [3–7]. With the development of imaging techniques and tools, PRB has improved the accuracy of sampling to higher than 95% and secured its safety, making it easily used in children [8].
The general indications for renal biopsy include recurrent gross hematuria (RGH) of unknown origin, combined hematuria and proteinuria (CHP), and isolated proteinuria (IP). In addition, steroid-resistant nephrotic syndrome, acute kidney injury of unknown origin, unexplained chronic renal insufficiency, systemic disease with renal involvement, and renal transplant dysfunction are also common indications [9]. Although PRB is not usually indicated in children with isolated persistent microscopic hematuria (PMH), it warrants PRB if there is a family history of significant renal disease or the parents are anxious about the diagnosis and prognosis. However, some cases of renal disease are undetected despite proper specimen collection by PRB in accordance with its indication. Our study was conducted to help understand clinical outcomes by analyzing the clinical course of pediatric patients with normal PRB histopathological findings.
Methods
Patients and design
The medical records of 552 subjects who underwent PRB between 2005 and 2016 at the Department of Pediatrics, Kyungpook National University Hospital were reviewed, and 26 subjects were excluded due to previous allograft biopsy (n = 9) or age at biopsy greater than 18 years (n = 17). The patient’s age, sex, clinical symptoms, and laboratory findings at the first and last visits after PRB were retrospectively analyzed (Fig. 1). Proteinuria was defined by spot urine protein-to-creatinine ratio (UPCR) ≥ 0.2 in the IP patient group. Microscopic hematuria was defined as at least five red blood cells per high-power microscopic field in urinary sediment without gross hematuria. Although there is no agreement on the criteria to classify hematuria as glomerular or nonglomerular, we concluded that the hematuria was more likely to be of glomerular origin if the proportion of dysmorphic red blood cells was higher than 30%. The estimated glomerular filtration rate (eGFR) was calculated using the updated Schwartz formula [10]. For safety, laboratory tests, including hemoglobin and platelet counts, prothrombin time, and partial thromboplastin time, were performed prior to PRB. In addition, blood urea nitrogen; serum creatinine, protein, and albumin levels; electrolyte count; urine dipstick reagent test results; and UPCR were used as baseline parameters in cases of complications. Kidney ultrasonography was used to evaluate the following structural contraindications for PRB: multiple renal cysts, solitary kidney, acute pyelonephritis, perinephric abscess, and renal neoplasm. Medical problems such as uncontrolled bleeding diathesis, uncontrolled blood pressure, and severe obesity were also regarded as medical contraindications for PRB. Before PRB, the first morning urine sample was examined in all children who presented with IP to rule out orthostatic proteinuria. Serum calcium levels and spot urine calcium-to-creatinine ratios were measured in all children with hematuria to rule out idiopathic hypercalciuria. If patients showed exercise-related RGH, renal vein Doppler ultrasonography was performed to rule out nutcracker syndrome.
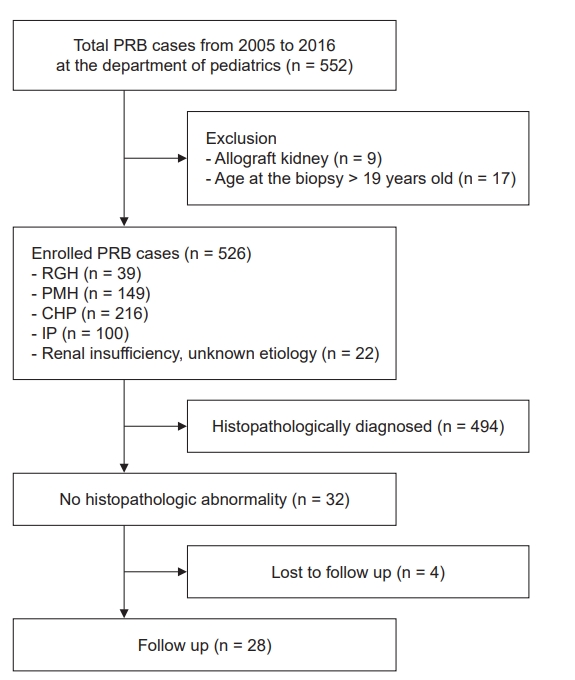
Flowchart of the study.
CHP, combined hematuria and proteinuria; eGFR, estimated glomerular filtration rate; IP, isolated proteinuria; PMH, isolated persistent microscopic hematuria; PRB, percutaneous renal biopsy.
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the requirement of informed consent was waived due to the retrospective nature of this study. This study was reviewed and approved by the Institutional Review Board of Kyungpook National University Hospital (No. 2021-04-003).
Biopsy procedure
PRB was performed by a pediatric nephrologist using a portable ultrasonography unit for localization. The patients were placed in a prone position and received local anesthesia or sedation. The biopsy was performed using a 16-gage (G) automated biopsy needle (16 G×150 mm, Acecut; TSK Laboratory, Tochigi, Japan) and repeated until specimen adequacy was confirmed by the pathologist. In native renal biopsies, the adequate yield of glomeruli is considered to be 10 to 20 when 14- and 16-G needles are used [5,11]. The sufficient specimens were divided for light microscopy (LM), immunofluorescence (IF), and electron microscopy (EM). For LM, the biopsy tissue was cut into 1- to 2-m-thick sections, fixed with 10% buffered formalin and embedded in paraffin. They were stained with hematoxylin-eosin, periodic acid-Schiff, Masson’s trichrome, and periodic acid-methenamine silver. The tissue was frozen for IF, and antibodies against immunoglobulin (Ig) G, IgA, IgM, complement C3, C1q, albumin, kappa light chains, and lambda light chains were used. With respect to EM, after prefixation with 2.5% glutaraldehyde, 0.1-M phosphate buffer was used. After fixation in 1% osmium tetroxide, the tissue was cut into 40- to 60-nm thick sections to measure the thickness of the glomerular basement membrane (GBM). Diagnostic criteria for thin basement membrane nephropathy (TBMN) according to age was based on the normal GBM thickness in a report of Japanese children (under 3 years old, <200 nm; 3–10 years old, <240 nm; above 10 years old, <300 nm) [12].
Statistics
Descriptive statistics were used to describe the data. Continuous variables were presented as mean ± standard deviation.
Results
All histopathological diagnoses in 526 patients are presented in Table 1. The most common indication of PRB in these children was CHP (n = 216, 41.1%), followed by PMH (n = 149, 28.3%), IP (n = 100, 19.0%), and RGH (n = 39, 7.4%). The most frequent histopathological diagnosis was TBMN (n = 136, 25.9%), followed by IgA nephropathy (n = 92, 17.5%), Henoch-Schönlein purpura nephritis (n = 73, 13.9%), and minimal change disease (MCD; n = 56, 10.6%).
Thirty-two out of 526 patients showed normal renal histopathological findings, accounting for 6.1% of the entire subject pool. Of these patients, 21 were male, and 11 were female (male-to-female ratio, 1.9:1). The mean ages at the first visit and at biopsy were 10.6 ± 4.1 and 11.4 ± 3.8 years, respectively (Table 2). The incidence rates of normal renal histopathology according to the indications for biopsy were as follows: RGH (9 of 39, 23.1%), PMH (10 of 149, 6.7%), IP (4 of 100, 4.0%), CHP (6 of 216, 2.8%), others (3 of 22, 13.6%), and total (32 of 526, 6.1%). The others indications for PRB were found in patients with renal insufficiency due to acute kidney injury of unknown origin, unexplained chronic renal insufficiency, or systemic disease with renal involvement. RGH showed the highest incidence rate for normal PRB findings, whereas CHP had the lowest incidence rate for normal renal histopathology among all the 526 subjects (Fig. 2).

Incidence rate of normal renal histopathology according to indications for biopsy
The dashed line represents the total incidence rate of normal renal histopathology in the 526 subjects (32 of 526, 6.1%).
RGH, recurrent gross hematuria; PMH, isolated persistent microscopic hematuria; IP, Isolated proteinuria; CHP, combined hematuria and proteinuria.
In the 32 patients with normal renal histopathology, the chief complaint at the first visit was RGH in nine patients (28.1%), PMH in 10 (31.3%), CHP in 6 (18.8%), IP in 4 (12.5%), and other renal insufficiencies in 3 (9.4%), all of which were suitable indications for renal biopsy. In all 10 patients with CHP and IP, the amount of proteinuria was not in the nephrotic range. The mean eGFR at the first visit was 105.6 ± 36.2 mL/min/1.73 m2. As a result of PRB, the mean glomeruli yield for the LM was 15.7 ± 12.2, and all specimens were determined to be properly collected. No diagnostic abnormalities were found with LM, IF, and EM among all subjects with normal renal histopathology (Table 2).
The mean follow-up period was 35.5 ± 23.6 months after the first visit and 25.8 ± 20.5 months after the biopsy, excluding four subjects who were lost to follow-up, of whom one had RGH, two had PMH, and one had CHP. According to chief complaints of patients with normal PRB findings at their first visit, analysis at the last visit after biopsy finally confirmed the following symptoms and urine abnormalities. Among the eight subjects who presented RGH at the first visit, six (75.0%) had no gross or microscopic hematuria-related events during the follow-up period. Gross hematuria and microscopic hematuria were each confirmed in one subject (12.5% and 12.5%, respectively). Among the eight subjects that presented PMH, four (50.0%) showed improvement. Among the five subjects who presented CHP, four (80.0%) showed improvement in urine abnormalities, and one (20.0%) was subsequently diagnosed with PMH. Among the four subjects with IP at the time of their first visit, three (75.0%) were later found to have persistent IP. The mean UPCR of three subjects with persistent IP was 0.62 ± 0.30 at the first visit and 0.25 ± 0.17 after the biopsy. Among the three subjects who presented with renal insufficiency, there was no eGFR decline; their mean eGFR was 65.5 ± 10.6 mL/min/1.73 m2 at the first visit and 69.2 ± 4.9 mL/min/1.73 m2 after the biopsy. One had persistent renal insufficiency, but the other two showed improvement of symptoms (Table 3).
Of the 28 subjects with normal renal histopathological findings, none showed progression to end-stage renal disease (ESRD) or required rebiopsy due to worsening of symptoms during the follow-up period.
Discussion
PRB is a highly effective and relatively safe method for diagnosing renal disease and is widely used in children. As the PRB technique has gradually developed, the accuracy of sampling has increased, and the diagnostic rate has improved accordingly. The indications for PRB include RGH, PMH, CHP, IP, steroid-resistant nephrotic syndrome, unexplained renal insufficiency, systemic disease with renal involvement, and renal transplant dysfunction [9]. In fact, nephrotic syndrome in children is not an absolute indication for renal biopsy because most of these patients show MCD and good response to corticosteroid treatment, unlike adults. Therefore, PRB is indicated only in children with nephrotic syndrome who are steroid dependent or resistant.
Several studies have reported the use of PRB in children with normal histopathological findings [13–16]. On the basis of questionnaire survey results from 11 pediatric renal centers in the UK, Hussain et al. [13] reported that the most common indication for a native kidney biopsy in children was proteinuria, followed by nephrotic syndrome, acute renal failure, and isolated hematuria. In addition, they reported that 32 of 346 native kidney biopsies (9.2%) were classified as normal, with proteinuria, isolated hematuria, acute glomerulonephritis, and drug toxicity justifying the biopsies. Prada Rico et al. [14] reported that 22 of 241 native kidney biopsies (9.1%) in children presented normal or nonspecific histopathological findings. Scheckner et al. [15] reported that 21 of 196 kidney biopsies (10.7%) were nondiagnostic and useless for patient management. However, no previous study has reported the clinical manifestations and outcomes of pediatric patients with normal renal histopathology evaluated by PRB.
In this study, 32 of the 526 children (6.1%) who underwent PRB presented with normal pathological findings, and the indications for biopsy in these patients were as follows: RGH (9 of 32 patients, 28.1%), PMH with (6 of 32 patients, 18.8%) or without proteinuria (10 of 32 patients, 31.3%), IP (4 of 32 patients, 12.5%), and unexplained renal insufficiency (3 of 32 patients, 9.4%). In contrast, all the children with nephrotic syndrome and systemic disease with renal involvement presented significant histopathological PRB abnormalities. In this study, we explained the clinical manifestations and outcomes in children with normal renal PRB histopathology.
In this study, the most common indication for PRB in the children with normal kidney histopathology was isolated hematuria, RGH and/or PMH without other symptoms, such as proteinuria, edema, hypertension, or renal insufficiency (19 of 32 patients, 59.4%). In fact, many reports have presented evidence to support that the prognosis of isolated hematuria is good and that renal biopsy is not indicated for children with isolated hematuria [17–20]. Hisano et al. [17] reported that all 135 children with isolated microhematuria did not develop hypertension or renal impairment after a mean period of 7.4 years, and 35 children had normal urinary findings within 6 years of their initial visit. A Hungarian multicenter study reported that 47.8% of 341 children with isolated hematuria became symptom-free [18]. In children, IgA nephropathy and idiopathic hypercalciuria are the most common glomerular and nonglomerular causes of isolated hematuria, respectively. Therefore, the spot urine calcium-to-creatinine ratio was checked in all patients before PRB was performed.
In Korea, the number of children with microscopic hematuria has increased after annual school urine screening tests have been performed since 1998. Since then, the prevalence of TBMN has increased greatly and was reported to be the most common pathological finding in pediatric patients with asymptomatic hematuria [21]. Patients with TBMN generally show normal findings in LM and IF, and EM findings typically demonstrate diffuse and uniform GBM thinning; the prognosis of TBMN is good [21–23]. Therefore, isolated hematuria tends to be excluded among the indications for PRB in children. However, some clinicians offer different opinions. Although hematuria has long been considered a benign condition related to glomerular disease, Moreno et al. [24] suggested that glomerular hematuria may lead to persistent renal injury. In addition, Baek et al. [22] reported that at least 5% of children initially demonstrating TBMN go on to manifest the classical electron-microscopic findings of Alport syndrome during childhood, and episodes of gross hematuria with TBMN can be a significant clue for the progression of Alport syndrome. Finally, regular follow-up of renal function in children with isolated hematuria, if possible, combined with genetic studies may be recommended [24].
In this study, 10 of the 32 patients (31.3%) with normal kidney histopathology had proteinuria with or without PMH as the indication for PRB. Although many clinicians no longer recommend isolated low-grade proteinuria (150–1,000 mg/day) as an indication for renal biopsy, persistent asymptomatic proteinuria as a criterion for renal biopsy in children is still controversial [25]. Zhai et al. [20] suggested that most pediatric asymptomatic proteinuria patients with or without microscopic hematuria had chronic progressive glomerulonephritis, such as IgA nephropathy, focal segmental glomerulosclerosis, and Alport syndrome. Trachtman et al. [26] reported that 12 out of 17 children (70.6%) with IP presented significant pathological findings, such as focal segmental glomerulosclerosis and membranous nephropathy, and the level of proteinuria did not predict the presence or absence of important kidney disease. However, Hama et al. [25] suggested that the adequate renal biopsy criterion in children with asymptomatic constant IP is UPCR ≥ 0.5. They reported that the diagnostic rate of minor glomerular abnormalities was 14 out of 15 children (93.3%) with low-grade proteinuria (UPCR < 0.5) but 17 out of 29 children (58.6%) with higher-grade proteinuria (UPCR ≥ 0.5) [25].
In this study, the clinical outcomes of children with normal histopathological PRB findings were favorable. During the follow-up period (means of 35.5 ± 23.6 months after the first visit and 25.8 ± 20.5 months after biopsy), six out of eight subjects (75.0%) with RGH, four out of eight subjects (50.0%) with PMH, four out of five subjects (80.0%) with CHP, and one out of four subjects (25.0%) with IP showed improvement in urinary abnormalities. In addition, among the three subjects who presented with renal insufficiency without abnormal urine, one developed persistent renal insufficiency, but the other two showed improvement of renal insufficiency. Of the 28 subjects with normal renal histopathological findings, none showed progression to ESRD or required rebiopsy due to worsening of symptoms during the follow-up period.
Then, to explain abnormal urinalysis findings despite nondiagnostic renal pathology in children, we hypothesized two possible causes of these results: (1) pathological study was too early and (2) orthostatic proteinuria or nonglomerular causes such as idiopathic hypercalciuria or nutcracker syndrome were not ruled out in spite of evaluation before PRB. First, normal renal pathology in spite of abnormal urinalysis, so-called subpathological renal damage, would be possible during childhood. We suggest that school urinalysis screening, as in Korea, can provide early recognition of abnormal urinalysis; thus, it could contribute to the increase in subpathological status diagnosis rate by PRB. In addition, although examination of first morning urine samples to rule out orthostatic proteinuria and the measurement of spot urine calcium-to-creatinine ratio to rule out idiopathic hypercalciuria were performed, results sometimes fluctuated according to the sampling method used or the patient’s diet. Renal vein Doppler ultrasonography can also fail to diagnose nutcracker syndrome as a cause of RGH, especially in small children.
In summary, despite the appropriate application of renal biopsy, the incidence rate of normal renal histopathology in children was 6.1%, and clinical outcomes were good, as most urine abnormalities improved, and none of the patients showed progression to ESRD or required rebiopsy due to worsening of symptoms including aggravation of proteinuria, increase in frequency of gross hematuria and deterioration of renal function during the follow-up period. Further studies to evaluate the long-term outcomes of children with normal renal histopathology are needed.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Conceptualization: MHC
Data curation: MJP, HMJ, HSB, SIL, MHH, YJK
Investigation: NK, MJP, HMJ, HSB, SIL, MHH, YJK, MHC
Writing–original draft: NK, MHC
Writing–review & editing: All authors
All authors read and approved the final manuscript.



