Additive harmful effects of acute kidney injury and acute heart failure on mortality in hospitalized patients
Article information
Abstract
Background
Organ crosstalk between the kidney and the heart has been suggested. Acute kidney injury (AKI) and acute heart failure (AHF) are well-known independent risk factors for mortality in hospitalized patients. This study aimed to investigate if these conditions have an additive effect on mortality in hospitalized patients, as this has not been explored in previous studies.
Methods
We retrospectively reviewed the records of 101,804 hospitalized patients who visited two tertiary hospitals in the Republic of Korea over a period of 5 years. AKI was diagnosed using serum creatinine-based criteria, and AHF was classified using International Classification of Diseases codes within 2 weeks after admission. Patients were divided into four groups according to the two conditions. The primary outcome was all-cause mortality.
Results
AKI occurred in 6.8% of all patients (n = 6,920) and AHF in 1.2% (n = 1,244). Three hundred thirty-one patients (0.3%) developed both conditions while AKI alone was present in 6,589 patients (6.5%) and AHF alone in 913 patients (0.9%). Among the 5,181 patients (5.1%) who died, 20.8% died within 1 month. The hazard ratio for 1-month mortality was 29.23 in patients with both conditions, 15.00 for AKI only, and 3.39 for AHF only. The relative excess risk of interaction was 11.85 (95% confidence interval, 2.43‒21.27), and was more prominent in patients aged <75 years and those without chronic heart failure.
Conclusion
AKI and AHF have a detrimental additive effect on short-term mortality in hospitalized patients.
Introduction
Acute kidney injury (AKI) is an important clinical condition diagnosed in hospitalized patients over time that affects more than 20% of patients. It is consistently associated with high medical costs, prolonged hospital stay, and increased mortality [1–3]. AKI also contributes to a higher risk of long-term poor renal outcomes and cardiovascular events [4]. However, little is known about comorbidities that develop as complications of AKI and their clinical consequences.
Cardiorenal syndrome is the term used to describe coexisting cardiac and renal dysfunction [5]. Acute heart failure (AHF) also contributes to short-term mortality risk and economic burden in hospitalized patients [6,7]. AKI is associated with 58% of congestive heart failure cases, and, in one study, the risk of congestive heart failure itself was higher with increasing AKI severity [8]. Although numerous attempts have been made to determine the prognoses of patients with cardiorenal syndrome, especially with respect to AKI and AHF, such reports have been limited to specific groups [9–11]. Epidemiological investigation of cardiorenal syndrome is challenging due to dynamic changes in renal function [12], heterogeneity of causes of AKI [13], difficulty in clarifying the temporal association between AKI and acute cardiac events, and lack of longitudinal follow-up data regarding coexisting conditions after AKI. In one previous study, the causes of death in hospitalized patients with AKI were reported. The results showed that 19.7% of all patients died from cardiovascular disease, and 3.1% of them from AKI [14]. Despite these limitations, there is a need for large epidemiological studies as some factors that influence AKI or AHF are potentially reversible.
Patients with coexisting AKI and AHF share many risk factors and medical treatments. This study aimed to investigate the interaction between AKI and AHF on death in hospitalized patients and find ways to improve mortality in this group of patients by focusing on reversible risk factors.
Methods
Study population and data sources
The study cohort was derived retrospectively from Seoul National University Hospital and Seoul National University Bundang Hospital in the Republic of Korea. We retrospectively collected data on the demographic and clinical characteristics of all patients over 18 years old who were admitted to either of the hospitals at least once from January 1, 2013 to December 31, 2017. Total follow-up time for all patients was about 7 years, from January 1, 2013 to October 30, 2019.
Patients with at least two serum creatinine measurements within 7 days of admission were considered eligible for inclusion in the study. Patients who started renal replacement therapy before the first admission were excluded. Clinical data collection was based on electronic medical record (EMR) system review. Information about the development of end-stage renal disease (ESRD) was obtained from the EMR system of each hospital and the ESRD registry of the Korea Society of Nephrology. Information about death was collected from the EMR system of each hospital and the Ministry of the Interior and Safety database by comparing the name and date of birth of each patient. Follow-up data were collected until October 30, 2019.
Assessment of kidney function
Definition of AKI was based on the Kidney Disease Improving Global Outcomes (KDIGO) criteria of creatinine measurement because urine output measurements were not available for all hospitalized patients. In more detail, AKI was diagnosed based on the change in serum creatinine concentration from the baseline value at the first measurement during admission to the peak level observed within 7 days after admission. The change in creatinine concentration was categorized according to the KDIGO criteria: AKI stage 1, ≥0.3 mg/dL absolute or 1.5- to 2.0-fold relative increase in serum creatinine; AKI stage 2, >2- to 3-fold increase in serum creatinine; AKI stage 3, >3-fold increase in serum creatinine or serum creatinine ≥4.0 mg/dL with an acute rise of >0.5 mg/dL. Initiation of renal replacement therapy was classified as stage 3 AKI.
Outcomes
The primary outcome was all-cause mortality within 1 month. Mortality data were collected from the EMR system of each hospital and the Ministry of the Interior and Safety database by comparing the name and date of birth of each patient. ESRD was also investigated as an outcome. The incidence of ESRD was obtained from the ESRD registry of the Korea Society of Nephrology.
Exposure
Exposure was defined as development of AKI, AHF, or both. We assigned patients into four groups: a) patients with neither AKI nor incident AHF during the observational period; b) patients with AHF during the observational period but without AKI; c) patients with AKI but without incident AHF; and d) patients with incident AHF and AKI. AHF was clinically diagnosed based on International Classification of Disease-10 (ICD-10) codes. Based on the ICD-10 codes for heart failure, we defined AHF as the first diagnosis of heart failure within 2 weeks after admission during the observational period. Patients with a diagnosis of heart failure based on ICD-10 codes before admission were classified as having chronic heart failure (CHF). We collected data for patients with ICD-10 codes I110, I119, I130, I500, I5000‒5004, I5008, I501, I509, J81, J810, and R570, which included diagnosis of acute pulmonary edema, cardiogenic pulmonary edema, cardiogenic shock, chronic pulmonary edema, combined systolic and diastolic heart failure, congestive heart failure, diastolic, congestive heart failure, systolic dysfunction, diastolic heart failure, heart failure, hypertensive heart and renal disease with congestive heart failure, hypertensive heart disease, hypertensive heart failure with congestive heart failure, hypertensive heart failure without congestive heart failure, hypertensive heart failure, hypertensive heart failure with renal disease, left ventricular dysfunction, left ventricular failure, right heart failure, right ventricular dysfunction, right ventricular failure, systolic heart failure, or ventricular dysfunction.
Covariates
The following data were obtained on the first day of admission during data collection; age, sex, body weight, body mass index (BMI), comorbidities (ischemic heart disease, hypertension, diabetes mellitus, heart failure, liver disease, cerebrovascular disease, chronic obstructive pulmonary disease, chronic kidney injury, and cancer), history of AKI, medications used in the last 6 months before admission (acyclovir, beta blockers, calcium-channel blockers, diuretics, nonsteroidal anti-inflammatory drugs [NSAIDs], renin-angiotensin system blockers, statins, vancomycin, vasopressors, cisplatin, cyclosporine, colistin, and amphotericin), Charlson comorbidity score, use of a mechanical ventilator, admission duration, laboratory results on the first day of admission (serum creatinine, hemoglobin, albumin, bilirubin, calcium, glucose, potassium, chloride, gamma oxalate transaminase, gamma pyruvate transaminase, blood urea nitrogen, total carbon dioxide, platelet, and white blood cell counts), major operation, and minor operation. We classified surgery into two categories—1) over 1 hour as a major surgery and 2) less than 1 hour as a minor surgery—using operation names based on the expected time of each surgery. The expected time of each surgery was from the data used in previous study [15], which had developed postoperative AKI prediction score.
We used the abbreviated Modification of Diet in Renal Disease equation to estimate the glomerular filtration rate. The average serum creatinine on the first day of admission was used if serum creatinine was measured several times. Data about comorbid conditions were collected by reviewing the EMR system. Charlson comorbidity score was calculated using the name of the underlying disease based on the ICD-9-CM codes and corresponding ICD-10-AM codes [16].
Statistical analysis
Summary statistics are presented as percentages for categorical variables and means ± standard deviations for continuous variables. Each variable was compared between the two groups using Student t-test for continuous variables and the chi-square test for categorical variables. Survival curves were estimated using the Kaplan-Meier method and compared by log-rank tests among patients according to the development of AKI and AHF. We used time-dependent Cox proportional hazards model to determine the adjusted association between death and AKI. We considered backward stepwise variable selection with p < 0.05 and conventional risk factors associated with AKI, AHF, and death in hospitalized patients using collected sociodemographic variables to generate the final model. Thus, the final model was independent of demographic characteristics (age over 75 years, sex, and BMI), admission duration, comorbidities (hypertension, diabetes mellitus, chronic kidney disease [CKD], CHF, ischemic heart disease, liver disease, cerebrovascular disease, chronic obstructive pulmonary disease, and cancer), and medications used during the 6 months preceding admission (diuretics, renin-angiotensin system blockers, beta blockers, calcium-channel blockers, NSAIDs, vancomycin, and vasopressors). Plots of log (–log [survival function]) vs. log (follow-up time in years) were used to check the proportional hazards assumption for categorical variables. We further used relative excess risk due to interaction (RERI), attributable proportion due to interaction (AP), and the synergy index (SI) to assess the additive interaction between AKI and AHF. RERI was calculated as hazard ratio (HR, both AKI and AHF) − HR (AKI alone) − HR (AHF alone) + 1 [17,18]. AP was calculated as RERI divided by HR (both AKI and AHF). SI was calculated as the ratio of increase in HR due to exposure to both AKI and AHF to the sum of the increases due to exposure to each condition alone. RERI represented excess risk due to the interaction between AKI and AHF relative to the risk without exposure. AP referred to the attributable proportion of disease due to this interaction with exposure to both conditions. RERI of >0 or AP of >0 indicated a significant additive interaction. All statistical analyses were performed using STATA/SE version 16 (StataCorp LP., College Station, TX, USA) and R software version 4.0.4 (R Foundation, Vienna, Austria). For subgroup analysis, we divided patients by age, sex, underlying CHF and CKD, and compared HRs of 1-month mortality according to the presence of AKI or AHF in each subgroup. A p-value of < 0.05 was considered significant. All probabilities were two-sided.
Ethical approval
This study was performed in accordance with the Declaration of Helsinki. The study protocol was approved by the Institutional Review Boards of Seoul National University Bundang Hospital (B-1910/570-404) and Seoul National University Hospital (H-1909-079-1064). Informed consent was waived because of the retrospective nature of the study.
Results
Baseline characteristics
Among 101,804 patients included in the study, the mean age was 58 ± 16.7 years, and 53,119 patients (52.2%) were male (Table 1). Percentages of patients with comorbidities were as follows: diabetes mellitus, 9.6%; hypertension, 12.3%; ischemic heart disease, 9.0%; heart failure, 1.8%; CKD, 2.0%; cerebrovascular disease, 13.8%; and cancer, 26.4%. De novo heart failure or acute aggravation of CHF occurred in 4,070 patients (4.0%) during the observational period and 1,244 patients (1.2%) developed de novo heart failure or acute CHF aggravation within 2 weeks. Cancer was present in 26,898 (26.4%) patients, and 507 (0.5%) patients experienced AKI before admission.
There were 6,589 patients (6.5%) with AKI only, 913 patients (0.9%) with AHF only, and 331 patients (0.3%) with both AKI and AHF. There were more elderly individuals among patients who developed AHF than patients without AHF, regardless of AKI. Patients with AHF had a higher proportion of underlying diseases, such as hypertension, ischemic heart disease, and CHF, and used diuretics more than those without AHF. Regardless of AKI, less than 20% of patients with AHF used NSAIDs compared to over 20% of those without AHF. Patients with AKI had longer hospital stays than those without AKI. In contrast, the presence of AHF did not significantly affect the length of hospital stay, even among patients who developed AKI. The percentages of patients with AKI who used beta blockers, renin-angiotensin system blockers, and calcium-channel blockers were similar regardless of the development of AHF. Usage proportion of vasopressors was higher among patients with AKI than those without AKI.
The highest proportion of CKD was found among patients who had both AKI and AHF (15.7%). One hundred sixty-eight patients (50.8%) with both AKI and AHF were admitted to the intensive care unit, and this proportion was higher than that of patients with either AKI or AHF. Serum potassium on the first day of admission was higher among patients with AKI than those without AKI, and it was highest in patients who developed both AKI and AHF.
Additive effect of acute kidney injury and acute heart failure
The overall death rate was 5.1% (5,181 patients), and 18.7% (121 patients) of patients who died developed both AKI and AHF. Among patients with AHF only, 350 (10.2%) died, while 933 patients (14.8%) with AKI only died (p < 0.001). Kaplan-Meier analysis showed a significant relationship between mortality and development of AKI or AHF based on a log-rank test (p < 0.001) (Fig. 1). As mortality rate was significantly higher early after enrollment, we further investigated 1-month mortality.
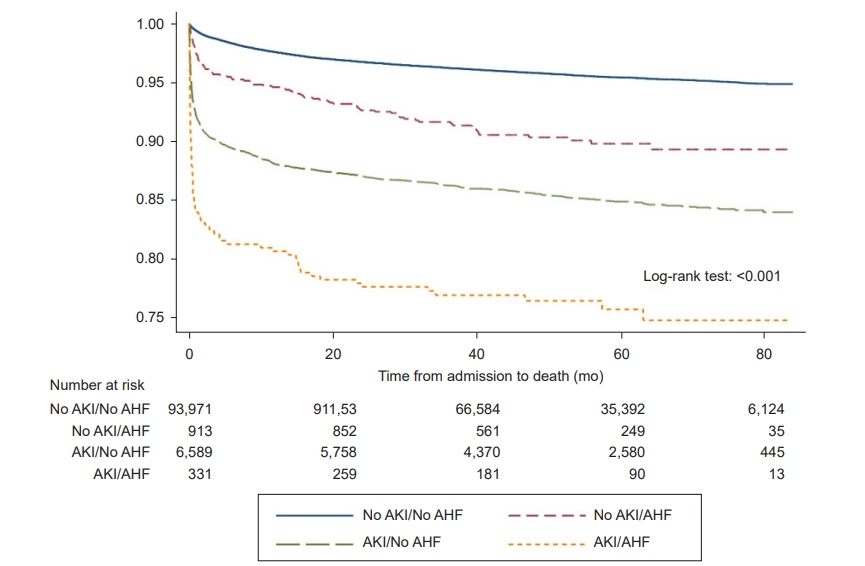
Kaplan-Meier curves for death by groups, based on the presence of AKI or AHF.
The coexistence of AKI and AHF increased the risk of mortality and end-stage renal disease, especially short-term mortality after admission.
AKI, acute kidney injury; AHF, acute heart failure.
Within 1 month after admission, 1,076 patients (20.8%) died. Among these patients, 4.8% (52 patients) developed both AKI and AHF (Table 2). We compared the HRs of patients according to AKI or AHF to that of patients without AKI or AHF. The HR of 1-month mortality was 29.23 in patients with both AKI and AHF (95% confidence interval [CI], 20.83‒41.03), while it was 15.00 in patients with AKI only (95% CI, 13.09‒17.19) and 3.39 in patients with AHF only (95% CI, 2.10‒5.47). Within strata of AHF, the HRs of AKI to 1-month mortality did not differ substantially. Interaction indexes in RERI analysis suggested that AKI and AHF had an additive detrimental effect on 1-month mortality; RERI between AKI and AHF was 11.846 (p = 0.014), AP was 0.405 (p < 0.001), and SI was 1.723 (p = 0.002). After 1 month, the additive detrimental effect on mortality was not significant.
There were 1,031 patients (1.0%) who developed ESRD after the first admission within the observational period. Among them, 51 patients (4.9%) developed both AKI and AHF, 661 patients (64.1%) developed AKI only, and eight patients (0.8%) developed AHF only. AKI increased the risk of ESRD by 19.120-fold, and AHF increased the risk of ESRD by 1.560-fold. In RERI analysis, the HR of 1-month mortality was 28.599 in patients with both AKI and AHF (95% CI, 20.17‒40.55). However, the additive effect of AKI and AHF on ESRD were not statistically significant (RERI: 8.057, p = 0.10; AP: 0.282, p = 0.022; S: 1.412, p = 0.05).
Subgroup analysis
In subgroup analysis, age was related to the adverse additive effect of AKI and AHF on short-term mortality. When patients were grouped by age, HRs in patients aged ≤75 years were higher than HRs in the total patient population (vs. patients without AKI or AHF: HR, 70.40 [95% CI, 41.85‒118.46] in patients with AKI and AHF; HR, 18.74 [95% CI, 15.62‒22.47] in patients with AKI only; and HR, 5.63 [95% CI, 2.42‒13.11] in patients with AHF only) (Table 3). RERI was 47.042 (p = 0.009), and AP and SI were also significant (AP, 0.668 [p < 0.001]; SI, 3.104 [p < 0.001]). In contrast, in patients aged over 75 years, RERI was 2.428 (p = 0.43), AP was 0.170 (p = 0.35), and SI was 1.224 (p = 0.39), which were statistically insignificant (Table 4). In elderly patients, 1-month mortality was higher in patients with AKI and AHF, with AKI only, and with AHF only (vs. patients without AKI or AHF: HR, 14.29 [95% CI, 9.16‒22.29] in patients with AKI and AHF; HR, 10.76 [95% CI, 8.77‒13.20] in patients with AKI only; and HR, 2.11 [95% CI, 1.18‒3.77] in patients with AHF only).
CHF and renal dysfunction were related to the HRs of AKI and AHF to 1-month mortality in subgroup analysis. Patients without CHF had a 43.736-fold increased risk (95% CI, 30.06‒63.63) of 1-month mortality when both AKI and AHF developed (Fig. 2). Patients with CHF showed 10.031-fold increased 1-month mortality risk (95% CI, 4.75‒21.17) when both AKI and AHF developed, which was significantly lower than that in patients without CHF. Among patients with CHF, HR for patients with AKI only was higher than for patients who developed both AKI and AHF (HR, 10.66; 95% CI, 5.36‒21.20). Patients with baseline eGFR over 60 mL/min/1.73 m2 had a 26.756-fold risk of 1-month mortality (95% CI, 14.46‒49.50), while patients with baseline eGFR under 60 mL/min/1.73 m2 had a 17.115-fold risk (95% CI, 11.24‒26.07).
Hazard ratios of confounders in multivariable Cox proportional hazard analysis
In multivariable analysis, HR of 1-month mortality was 2.56 (95% CI, 2.28‒2.97) for patients aged >75 years compared with patients under 75 years (Fig. 3). Males had 1.367-fold increased risk of 1-month mortality than women (HR, 1.37; 95% CI, 1.22‒1.58). BMI was associated with 1-month mortality as well (HR, 0.87; 95% CI, 0.86‒0.89]). Previous use of diuretics and vasopressor had HRs of 1.64 (95% CI, 1.31‒2.05) and 2.52 (95% CI, 1.96‒3.25), respectively. Previous use of beta blockers was also significant (HR, 0.63; 95% CI, 0.46‒0.87). Comorbidities were associated with 1-month mortality as follows: HR, 2.00 (95% CI, 1.44‒2.79) for liver disease; HR, 1.40 (95% CI, 1.18‒1.66) for cerebrovascular disease; HR, 1.38 (95% CI, 1.21‒1.59) for cancer; HR, 1.31 (95% CI, 1.07‒1.60) for ischemic heart disease; HR, 0.77 (95% CI, 0.62‒0.96) for diabetes mellitus; and HR, 0.541 (95% CI, 0.39‒0.75) for CKD.
Discussion
This study demonstrated that AKI and AHF have additive harmful effects on 1-month mortality. The coexistence of AKI and AHF increased mortality risk more than isolated organ dysfunction in patients without CHF and CKD, especially non-elderly patients.
Four types of cardiorenal syndrome are currently recognized based on the primary organ defect detected first [19]. Coexisting AKI and AHF may complement each other and interact synergistically to produce other risk factors for mortality. Kidney dysfunction can induce heart apoptosis [20], and heart dysfunction can also change kidney structure [21]. Current temporal classification is not perfectly explained by our current pathophysiological understanding of the organ crosstalk between the heart and kidney. In this study, we investigated AKI and AHF that developed within a short period after admission without considering the temporal relationship between AKI and AHF and found that these two conditions had an additive detrimental effect on short-term mortality. A previous study of patients who started renal replacement therapy suggested that heart failure impeded recovery from AKI [22]. Therefore, finding a way to detect dysfunction of these two organs is important.
In our study, the interactive effect of AHF and AKI on mortality lasted for a short period. A previous prospective cohort study reported that the association between AKI and mortality decreased after kidney function recovery and proteinuria at the three-month follow-up [23]. These results thus emphasize the need for early detection of and prompt intervention for AKI or AHF after admission, even in patients without previous chronic dysfunction of the heart or kidney. Availability of early diagnostic biomarkers of AKI that correlate with cardiovascular risk or mortality [24] and the development of predictive and prognostic algorithms by machine learning [25] will hopefully result in improved patient outcomes.
The causes of AKI are multifactorial and, similarly, AHF has a range of risk factors. This makes their relationship with acute mortality more challenging to discern, but it does not detract from their clinical significance during admission [26]. In patients older than 75 years in our study, there was an obscure interactive effect between AHF and AKI. We believe several other comorbidities that contribute to increased risk of mortality were present. A previous study suggested multiple organ dysfunction as a risk factor for mortality in elderly patients with AKI [27]. The results of our study also indicate that dysfunction of individual organs contributes to increased mortality.
Interestingly, the additive effect of AKI and AHF was more prominent in patients without CHF than in those with CHF. CHF was present in about 60% of patients who developed AKI and AHF. Patients with CHF who had either AKI or AHF had a lower HR of 1-month mortality than those without CHF. Management of CHF includes medical treatment with renin-angiotensin system blockers, beta blockers, and diuretics [28,29]. Because the pathophysiologic mechanisms of cardiorenal syndrome, involving AKI and AHF, are explained mostly by volume congestion [30–32], furosemide is one of the standard medications for managing volume congestion in AHF, and has been suggested to improve prognosis in AHF [33–35]. One study in patients with AKI suggested that furosemide reduced 2-month mortality when it was appropriately used for volume management [36]. Because de novo heart failure is usually associated with cardiogenic shock or severe hemodynamic alterations secondary to an insult, such as myocardial infarction (which is itself a risk factor for death [37]), usage of diuretics may be difficult. The consequent volume congestion would result in AKI and increased mortality [38].
We also found that the interactive effect of AKI and AHF was more prominent in patients without CKD than those with CKD. In our study, the proportion of patients with CKD among those who developed both AKI and AHF was 15.7%, while it was only 2.1% among patients who developed AHF. Progressive renal insufficiency might have led to acute decompensated heart failure and therefore admission to the hospital. However, the mortality of patients whose baseline eGFR was <60 mL/min/1.73 m2 was less affected by the additive effect of AKI and AHF. Because of the prevalent use of renin-angiotensin system blockers and/or angiotensin-converting enzyme inhibitors in patients with CKD [39], these drugs may also have exerted protective effects in patients with an ischemic insult to the heart, as a previous study suggested [40].
The limitations of this study are as follows. First, we based our clinical diagnoses on ICD codes, and heart function was not objectively assessed. Because the diagnosis of AHF depends on clinical judgment, mostly based on fluid congestion, prospective studies that utilize objective measurements of heart function would be useful to develop treatments for cardiorenal syndrome [41]. Second, we did not know the cause of admission or the etiology of AKI in the study patients. Because data on the specific etiology of AKI were limited, pathophysiological links between AKI and AHF could not be determined. However, analyzing large numbers of hospitalized patients would be a good starting point for studying cardiorenal syndrome and obtaining temporal evidence of organ crosstalk between AKI and AHF.
In conclusion, we studied the interaction between AKI combined with AHF and mortality. HRs of short-term mortality were elevated when AKI and AHF were both present. RERI suggested the possibility of a causal association of AKI and AHF with 1-month mortality, indicating the need for additional research. The coexistence of AKI and AHF in hospitalized patients was associated with increased mortality, even in patients whose renal function was near to normal. Great care must be taken to reverse fluid congestion and insults to the heart and kidney without causing additional renal impairment in these patients.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Conceptualization: SK
Study design: JYR, EB
Data curation, Formal analysis: HES, SSH, SK
Investigation: SSH, JJM, JP, HES
Supervision: SK, SSH, JCJ, HJC, KYN, DC
Writing–original draft: HES
Writing–review & editing: HES, SSH, SK
All authors read and approved the final manuscript.






