| Kidney Res Clin Pract > Volume 41(5); 2022 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This research was supported by grants (2014-ER6301-00, 2014-ER6301-01, 2014-ER6301-02, 2017-ER6301-00, 2017-ER6301-01, 2017-ER6301-02, and 2020-ER7201-00) from the Research of Korea Disease Control and Prevention Agency.
AuthorsŌĆÖ contributions
Conceptualization: MSK, DGK
Data curation: All authors
Formal analysis: All authors
Funding acquisition: YHP, JBP, SHL, JY, MSK, DGK
Investigation: YHP, JBP, SHL, JY, MSK, DGK
Methodology: All authors
Project administration: JYL, DGK
Visualization: SHK, DGK
WritingŌĆōoriginal draft: JYL, DGK
WritingŌĆōreview & editing: All authors
All authors read and approved the final manuscript.
Acknowledgments
Supplementary Materials
Figure┬Ā1.
Study population.
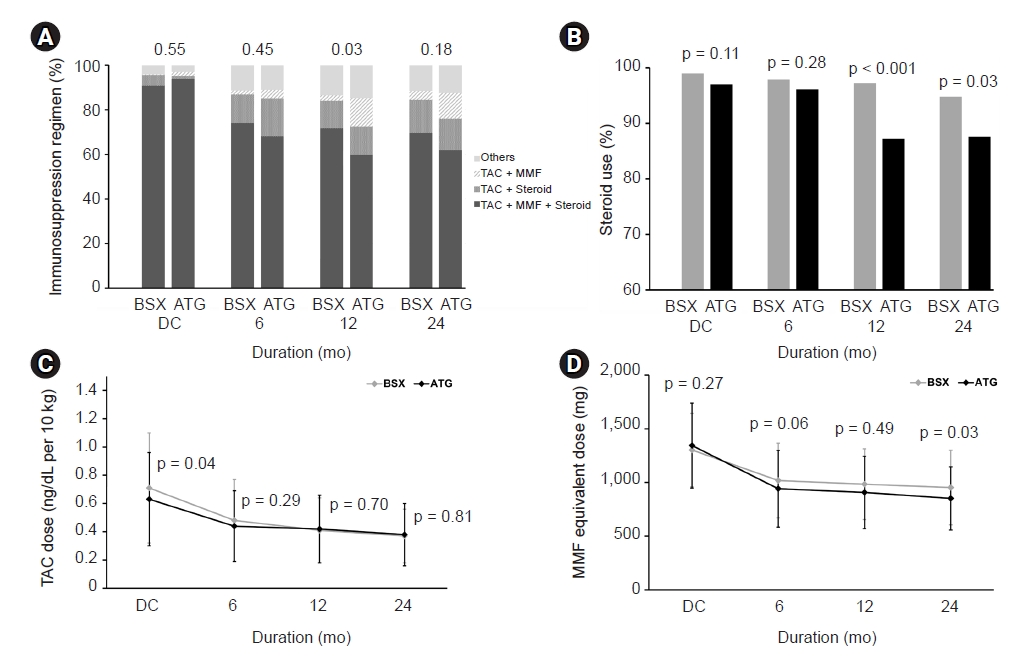
Figure┬Ā2.
Comparison of maintenance immunosuppression regimen between BSX and ATG group.
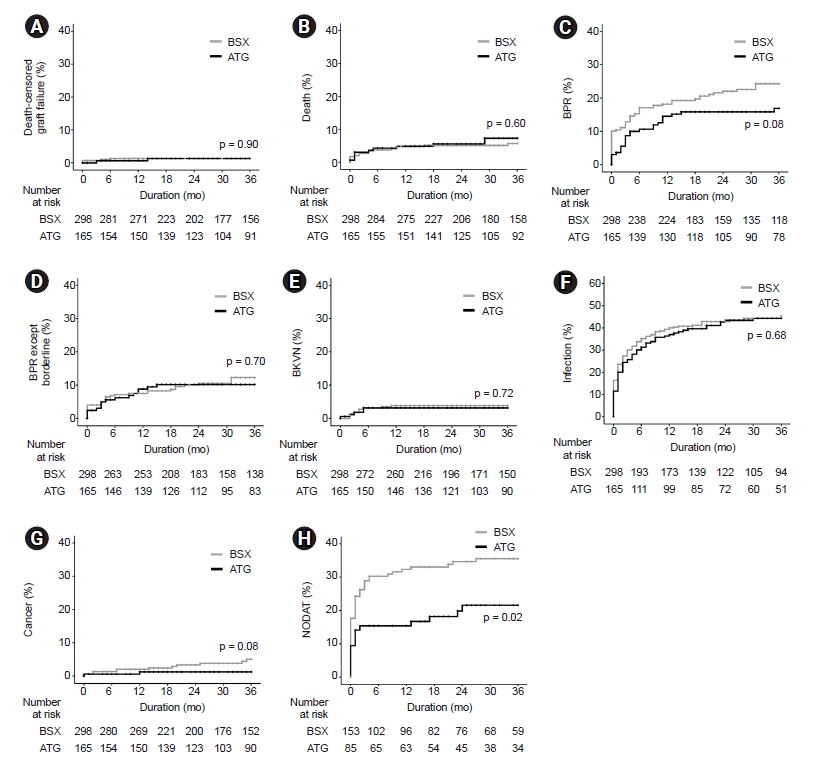
Figure┬Ā3.
Matched comparison of posttransplant events between ATG and BSX groups.
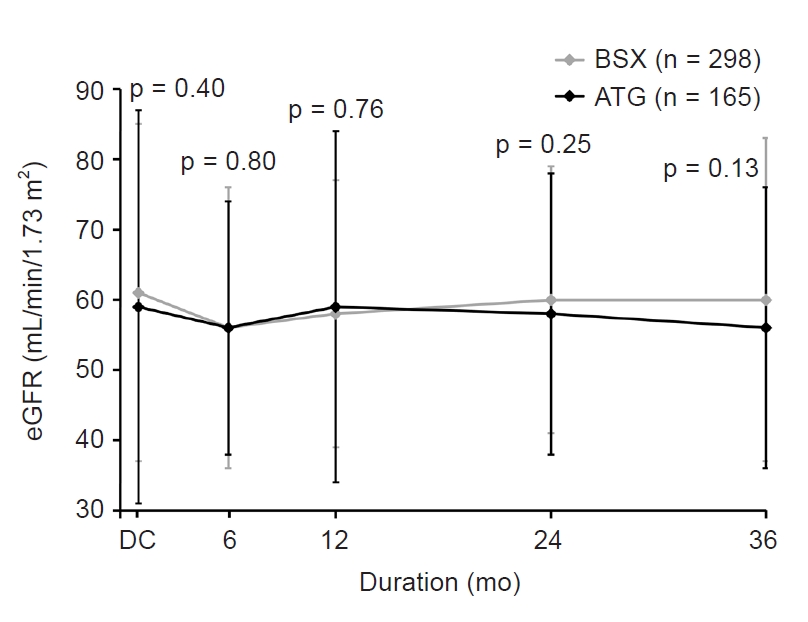
Figure┬Ā4.
Matched comparison of posttransplant graft function between ATG and BSX groups.
Table┬Ā1.
| Variable |
Before matching |
After matching |
||||
|---|---|---|---|---|---|---|
| BSX BSX (n = 656) | ATG (n = 191) | p-value | BSX (n = 298) | ATG (n = 165) | p-value | |
| Age (yr) | 64.3 ┬▒ 3.7 | 64.3 ┬▒ 3.3 | 0.884 | 64.2 ┬▒ 3.6 | 64.3 ┬▒ 3.4 | 0.41 |
| Male sex | 447 (68.1) | 129 (67.5) | 0.875 | 196 (65.8) | 113 (68.5) | 0.73 |
| BMI (kg/m2) | 23.6 ┬▒ 3.1 | 23.2 ┬▒ 2.7 | 0.187 | 23.1 ┬▒ 3.0 | 23.1 ┬▒ 2.8 | 0.55 |
| Year of KT | 0.767 | 0.62 | ||||
| ŌĆā2014ŌĆō2016 | 243 (37.0) | 73 (38.2) | 119 (39.9) | 62 (37.6) | ||
| ŌĆā2017ŌĆō2019 | 413 (63.0) | 118 (61.8) | 179 (60.1) | 103 (62.4) | ||
| Retransplantation | 19 (2.9) | 19 (9.9) | <0.001 | 17 (5.7) | 11 (6.7) | 0.40 |
| Cause of ESRD | 0.466 | 0.455 | ||||
| ŌĆāDM | 236 (36.0) | 75 (39.3) | 108 (36.2) | 66 (40.0) | ||
| ŌĆāHypertension | 112 (17.1) | 36 (18.8) | 45 (15.1) | 32 (19.4) | ||
| ŌĆāGlomerular disease | 132 (20.1) | 42 (22.0) | 67 (22.5) | 36 (21.8) | ||
| ŌĆāPCKD | 34 (5.2) | 10 (5.2) | 17 (5.7) | 9 (5.5) | ||
| ŌĆāOther disease | 13 (2.0) | 3 (1.6) | 7 (2.3) | 3 (1.8) | ||
| ŌĆāUnknown | 129 (19.6) | 25 (13.1) | 54 (18.2) | 19 (11.5) | ||
| Dialysis duration (mo) | 43.5 ┬▒ 51.0 | 61.4 ┬▒ 52.4 | <0.001 | 60.1 ┬▒ 51.7 | 61.2 ┬▒ 54.6 | 0.32 |
| DM | 317 (48.3) | 92 (48.2) | 0.97 | 145 (48.7) | 80 (48.5) | 0.94 |
| CVD | 161 (24.5) | 40 (20.9) | 0.303 | 64 (21.5) | 33 (20.0) | 0.54 |
| HLA mismatch | 3.5 ┬▒ 1.7 | 3.8 ┬▒ 1.7 | 0.107 | 3.8 ┬▒ 1.8 | 3.7 ┬▒ 1.7 | 0.60 |
| PRA, percentile groups | 0.003 | 0.94 | ||||
| ŌĆā0ŌĆō20 | 379 (57.8) | 92 (48.2) | 145 (48.7) | 82 (49.7) | ||
| ŌĆā21ŌĆō80 | 87 (13.3) | 25 (13.1) | 43 (14.4) | 22 (13.3) | ||
| ŌĆā81ŌĆō100 | 9 (1.4) | 10 (5.2) | 6 (2.0) | 3 (1.8) | ||
| ŌĆāUnknown | 181 (27.5) | 64 (33.5) | 104 (34.9) | 58 (35.2) | ||
| Donor type | <0.001 | <0.001 | ||||
| ŌĆāLiving donor | 324 (49.4) | 33 (17.3) | 74 (24.8) | 33 (20.0) | ||
| ŌĆāDeceased donor | 332 (50.6) | 158 (82.7) | 224 (75.2) | 132 (80.0) | ||
| Donor age (yr) | 51.3 ┬▒ 14.1 | 53.2 ┬▒ 14.2 | 0.094 | 53.6 ┬▒ 14.2 | 53.7 ┬▒ 14.2 | 0.65 |
| Donor sex, male | 354 (54.0) | 130 (68.1) | 0.001 | 188 (63.1) | 112 (67.9) | 0.06 |
| Donor BMI (kg/m2) | 23.9 ┬▒ 3.4 | 23.9 ┬▒ 3.9 | 0.919 | 23.8 ┬▒ 3.5 | 24.0 ┬▒ 3.7 | 0.57 |
| Donor creatinine at donation (mg/dL) | 1.0 ┬▒ 0.8 | 2.0 ┬▒ 1.7 | <0.001 | 1.6 ┬▒ 1.0 | 1.7 ┬▒ 1.3 | <0.001 |
| Donor HTN | 150 (22.9) | 58 (30.4) | 0.034 | 86 (28.9) | 49 (29.7) | 0.54 |
| Donor DM | 60 (9.1) | 31 (16.2) | 0.005 | 45 (15.1) | 27 (16.4) | 0.34 |
| ECD | 167 (25.5) | 80 (41.9) | <0.001 | 118 (39.6) | 71 (43.0) | 0.47 |
| Vascular cause of death | 128/332 (38.6) | 55/158 (34.8) | 0.423 | 87/224 (38.8) | 43/132 (32.6) | 0.24 |
| CIT (min)a | 170.7 ┬▒ 156.7 | 259.0 ┬▒ 159.8 | <0.001 | 233.5 ┬▒ 161.3 | 251.2 ┬▒ 162.7 | 0.32 |
| DDKT CIT (min)b | 298.5 ┬▒ 145.5 | 296.9 ┬▒ 148.6 | 0.925 | 296.0 ┬▒ 141.9 | 294.1 ┬▒ 152.3 | 0.92 |
Data are expressed as mean ┬▒ standard deviation or number (%).
ATG, antithymocyte globulin; BMI, body mass index; BSX, basiliximab; CIT, cold ischemic time; CVD, cardiovascular disease; DDKT, deceased donor kidney transplant; DM, diabetes mellitus; ECD, expanded criteria donor; ESRD, end-stage renal disease; HLA, human leukocyte antigens; HTN, hypertension; KT, kidney transplant; PCKD, polycystic kidney disease; PRA, panel-reactive antibody.
Table┬Ā2.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Jun Young Lee

https://orcid.org/0000-0001-8047-4190Sung Hwa Kim

https://orcid.org/0000-0002-5482-1758Yeon Ho Park

https://orcid.org/0000-0003-1623-2167Jae Berm Park

https://orcid.org/0000-0001-9117-2278Su Hyung Lee

https://orcid.org/0000-0001-7963-8311Jaeseok Yang

https://orcid.org/0000-0002-5378-7797Myoung Soo Kim

https://orcid.org/0000-0002-8975-8381Deok Gie Kim

https://orcid.org/0000-0001-9653-926X - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















