A 40-year-old female patient with lupus-related end-stage renal disease received a preemptive living donor kidney transplant from her sister. Although graft function was well maintained under tacrolimus, prednisolone, and mycophenolate mofetil (MMF) maintenance therapy, the patient experienced an asymptomatic urinary tract infection. Escherichia coli was detected in urine culture. Oral third-generation cephalosporins were administered to the patient until discharge. Fourteen weeks after transplantation, the patient came to the emergency department complaining of right flank pain and fever. Laboratory tests showed hematuria, pyuria, bacteriuria, and leukocytosis, indicating a urinary tract infection. The patient was treated with antibiotics and discharged from the emergency department.
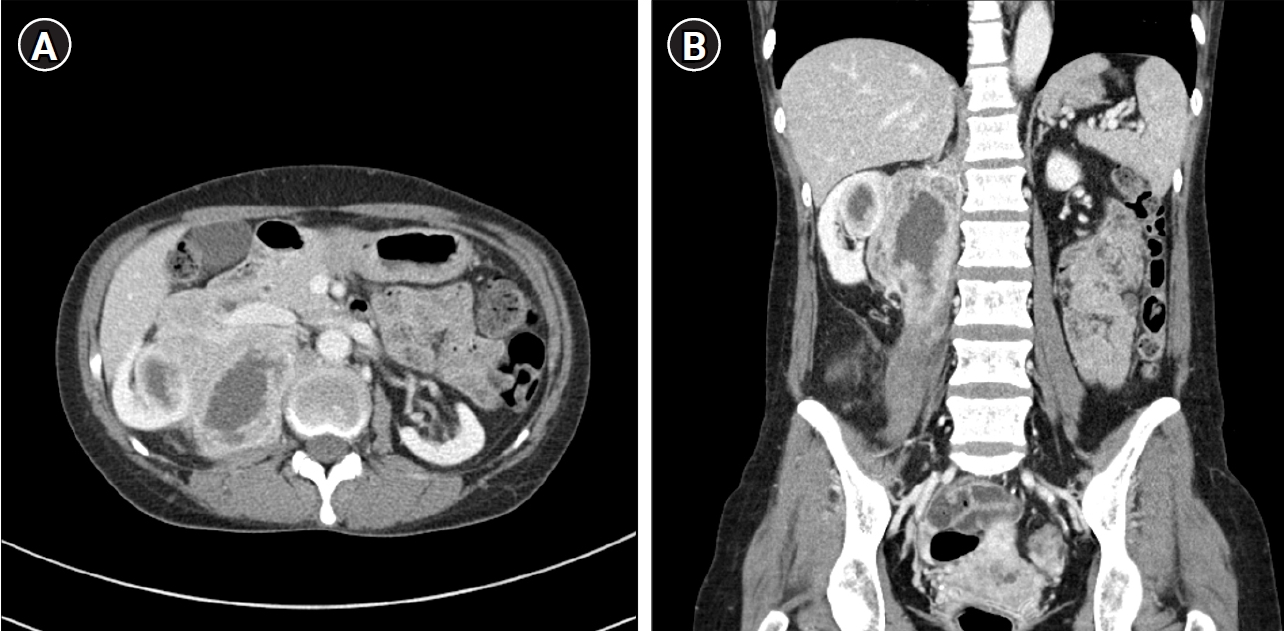
The patient revisited the emergency room with the same symptoms eighteen weeks after transplantation. At this time, there was no bacteriuria or pyuria on urinalysis. Computed tomography scan was performed to identify fever focus, and a huge infiltrative mass lesion was observed in the upper portion of the right native kidney, extending into the perinephric space and right psoas muscle (
Fig. 1). For diagnosis and treatment, ultrasound-guided gun biopsy was performed and percutaneous catheter drainage (PCD) was inserted.
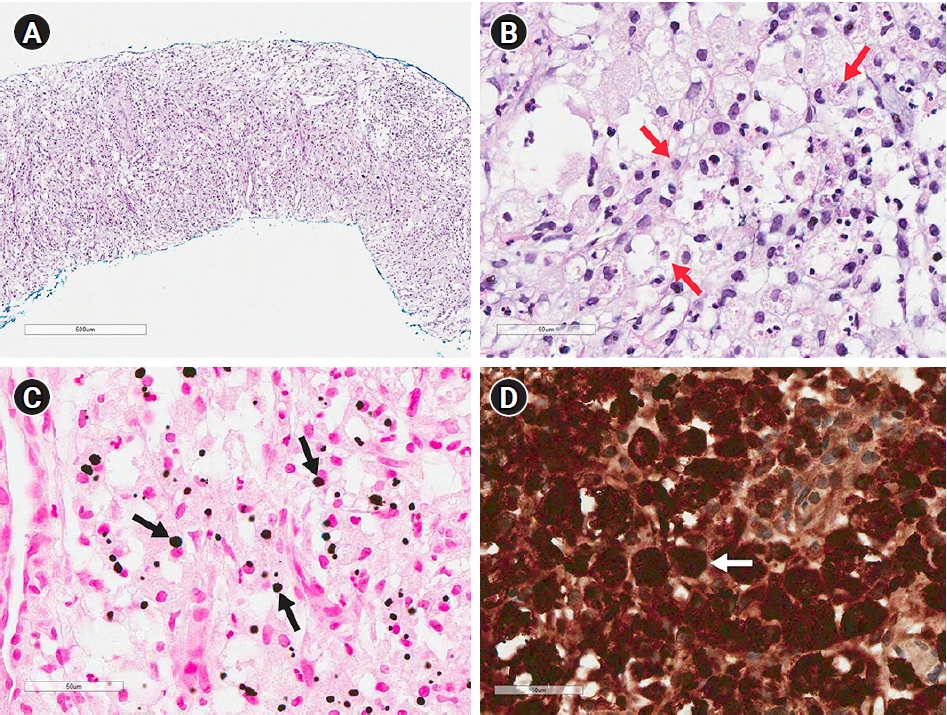
E. coli was identified on PCD culture, but there was no bacteria growth on urine culture. On microscopy, cellular infiltrates were predominantly composed of large macrophages with foamy granular eosinophilic cytoplasm, prominent eccentric, hyperchromatic and round nuclei, indicating von Hansemann cells. In von Kossa staining, the cells contained some intracytoplasmic, concentrically laminated, round-ovoid, basophilic inclusions called Michaelis-Gutmann bodies. In addition, CD68 was positive on immunohistochemical staining (
Fig. 2). These morphological and immunohistochemical characteristics were consistent with malakoplakia, not malignancy. Oral third-generation cephalosporin was administered to the patient for 4 months, and MMF was discontinued for 7 months. The patient recovered from malakoplakia without graft dysfunction or other complications.
Malakoplakia is an uncommon granulomatous inflammatory disorder that typically occurs in immunocompromised individuals. Malakoplakia affects mainly the genitourinary tract, although it involves naearly every organ. Malakoplakia of the graft kidney is more frequent in women with a history of bacterial urinary infection; E. coli is the most common pathogen. Almost all cases of kidney-related malakoplakia appear in transplanted kidneys and are associated with graft dysfunction. First-line treatment of malakoplakia involves long courses of antibiotics and a reduction in immunosuppression.
This is a rare case of post-transplantation malakoplakia that developed in the native kidney and not in the graft. We suggest that conventional treatment methods can effectively treat native renal malakoplakia without graft dysfunction.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















