| Kidney Res Clin Pract > Epub ahead of print |
|
Abstract
Background
Methods
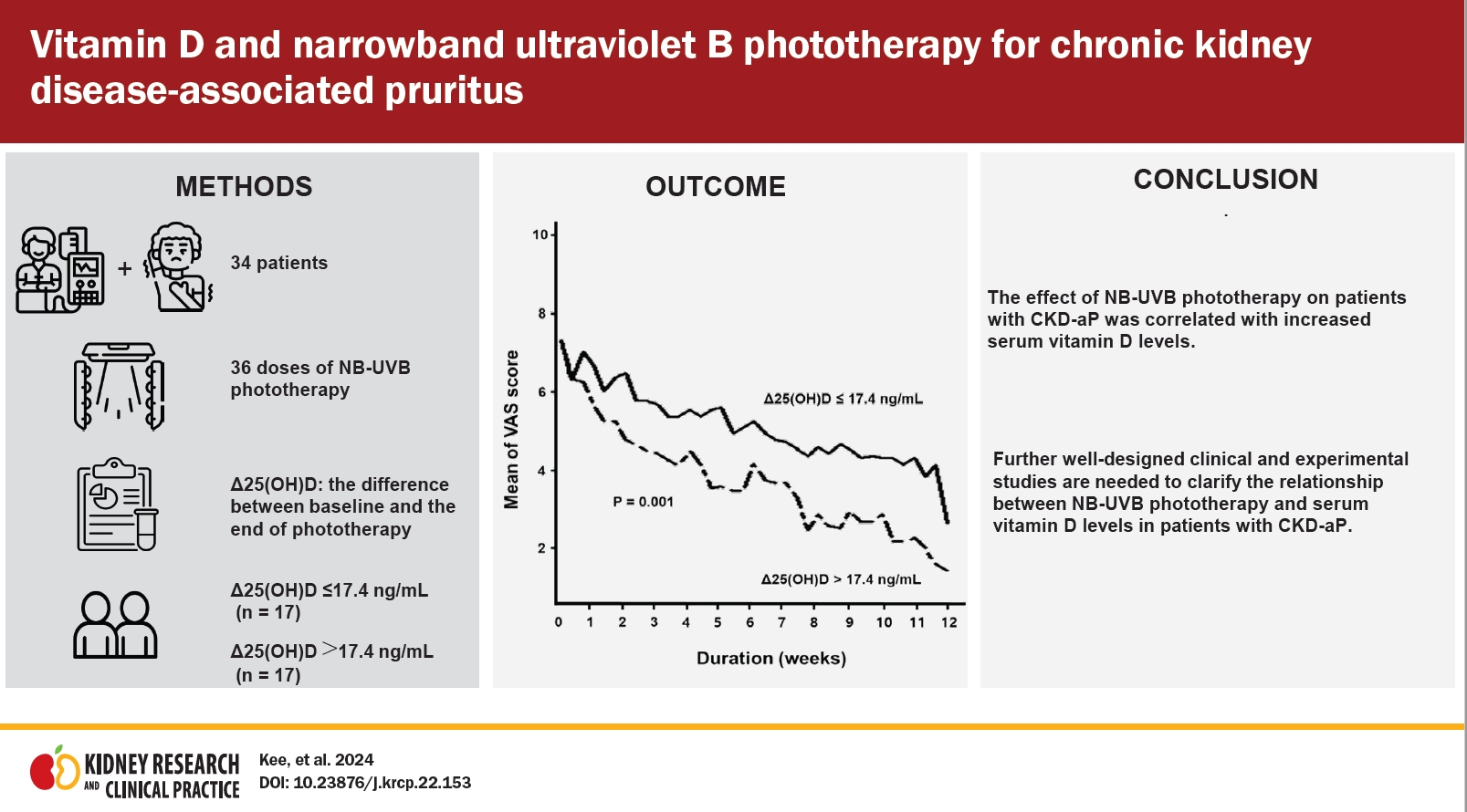
Results
Conclusion
Notes
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (NRF-2017R1C1B5074168).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization, Project administration: JO, DHS
Data curation: YKK, HJJ, JO
Formal analysis: YKK, HJJ
Investigation: HJJ
Methodology, Visualization: YKK
Supervision: DHS
Writing–original draft: YKK, DHS
Writing–review & editing: JO, DHS
All authors read and approved the final manuscript.
Acknowledgments
Figure 2.
Comparison of VAS scores of pruritus intensity over time during a course of narrowband ultraviolet B phototherapy between patients with Δ25(OH)D of >17.4 ng/mL and patients with Δ25(OH)D of ≤17.4 ng/mL.
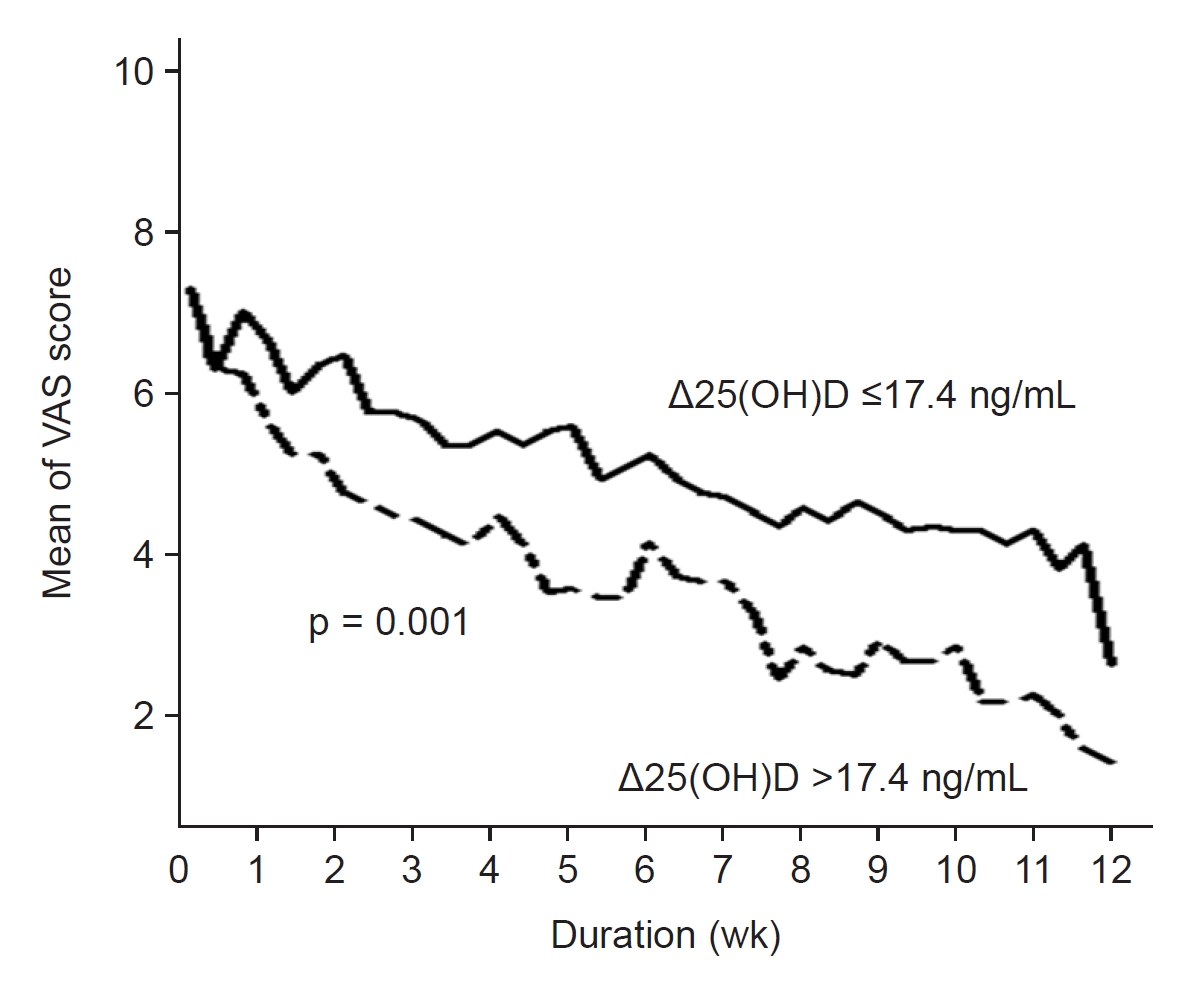
Table 1.
| Parameter | Before the first NB-UVB phototherapy | After the last NB-UVB phototherapy | p-value |
|---|---|---|---|
| Hemoglobin (g/dL) | 10.2 ± 0.7 | 10.1 ± 0.3 | 0.58 |
| Ferritin (ng/mL) | 126.5 (88.6–201.5) | 129.5 (97.8–190.5) | 0.96 |
| TSAT (%) | 26.7 (19.0–33.3) | 25.8 (18.9–34.9) | 0.30 |
| PTH (pg/mL) | 234.0 (162.0–468.8) | 319.0 (280.0–442.5) | 0.26 |
| 25(OH)D (ng/mL) | 10.9 (5.3–26.0) | 32.5 (25.8–39.3) | <0.001 |
| Calcium (mg/dL) | 8.7 ± 0.5 | 8.6 ± 0.5 | 0.16 |
| Phosphate (mg/dL) | 5.7 ± 1.5 | 5.6 ± 1.1 | 0.15 |
| CRP (mg/L) | 0.5 (0.5–1.3) | 0.5 (0.5–1.2) | 0.17 |
| Kt/Va | 1.5 ± 0.2 | 1.5 ± 0.1 | 0.22 |
Table 2.
| Characteristic | Total |
Δ25(OH)Da (ng/mL) |
p-value | |
|---|---|---|---|---|
| ≤17.4 | > 17.4 | |||
| Demographic data | ||||
| No. of patients | 34 | 17 | 17 | |
| Age (yr) | 62 (56–69) | 60 (55–65) | 63 (61–71) | 0.13 |
| Male sex | 14 (41.2) | 6 (35.3) | 8 (47.1) | 0.73 |
| Clinical data | ||||
| HD duration (mo) | 52.5 (29.6–107.4) | 54.6 (14.0–117.4) | 46.7 (37.3–55.0) | 0.56 |
| Underlying ESRD | ||||
| Diabetes | 23 (67.6) | 14 (82.4) | 9 (52.9) | 0.14 |
| Non-diabetes | 11 (32.4) | 3 (17.6) | 8 (47.1) | |
| VAS score | 7 (6–8) | 7 (6–8) | 7 (6–8) | >0.99 |
| Cumulative dose of NB-UVB phototherapy (mJ/cm2) | 44,010 (35,113–45,130) | 45,060 (35,130–45,130) | 43,940 (31,560–45,060) | 0.11 |
| Medication | ||||
| Vitamin D analogues | 13 (38.2) | 8 (47.1) | 5 (29.4) | 0.29 |
| Laboratory parameter | ||||
| Hemoglobin (g/dL) | 10.1 ± 0.3 | 10.1 ± 0.3 | 10.1 ± 0.2 | 0.72 |
| Ferritin (ng/mL) | 126.5 (88.8–201.5) | 139 (96 -224) | 117 (88–158) | 0.26 |
| TSAT (%) | 26.7 (19.0–33.3) | 27.3 (20.7–35.6) | 25.0 (16.9–31.3) | 0.35 |
| PTH (pg/mL) | 234.0 (162.0–468.8) | 414 (251–714) | 191 (132–220) | 0.001 |
| 25(OH)D (ng/mL) | 10.9 (5.28–26.0) | 15.0 (5.2–26.0) | 10.9 (5.3–25.0) | 0.52 |
| Calcium (mg/dL) | 8.7 ± 0.5 | 8.7 ± 0.4 | 8.8 ± 0.7 | 0.83 |
| Phosphate (mg/dL) | 5.7 ± 1.5 | 6.0 ± 1.8 | 5.5 ± 1.2 | 0.40 |
| CRP (mg/L) | 0.5 (0.5–1.3) | 1.0 (0.5–1.4) | 0.5 (0.5–1.1) | 0.22 |
| Kt/Vb | 1.5 ± 0.2 | 1.6 ± 0.2 | 1.5 ± 0.2 | 0.50 |
Data are expressed as number only, median (interquartile range), number (%), or mean ± standard deviation.
CRP, C-reactive protein; ESRD, end-stage renal disease; HD, hemodialysis; NB-UVB, narrowband ultraviolet B; TSAT, transferrin saturation; VAS, visual analog scale; 25(OH)D, 25-hydroxy vitamin D.
Table 3.
| Factor |
Univariate |
Multivariate |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | p-value | Odds ratio (95% CI) | p-value | |
| Age (yr) | 1.05 (0.97–1.13) | 0.23 | ||
| Male sex | 4.00 (0.70–22.90) | 0.12 | ||
| HD duration (mo) | 0.99 (0.97–1.01) | 0.37 | ||
| Diabetes | 2.40 (0.42–13.90) | 0.33 | ||
| Hemoglobin (g/dL) | 0.99 (0.07–14.35) | 0.99 | 0.46 (0.01–31.84) | 0.72 |
| Ferritin (ng/mL) | 1.00 (0.99–1.01) | 0.95 | 0.99 (0.98–1.01) | 0.62 |
| TSAT (%) | 1.01 (0.97–1.05) | 0.69 | 0.62 (0.91–1.17) | 0.62 |
| PTH (pg/mL) | 0.99 (0.99–1.00) | 0.17 | 1.00 (0.99–1.01) | 0.61 |
| 25(OH)D (ng/mL) | 1.01 (0.94–1.08) | 0.93 | 1.07 (0.94–1.22) | 0.28 |
| Δ25(OH)Da (ng/mL) | 1.20 (1.05–1.38) | 0.01 | 1.29 (1.02–1.63) | 0.04 |
| Calcium (mg/dL) | 1.41 (0.34–5.86) | 0.64 | 2.56 (0.18–36.80) | 0.49 |
| Phosphate (mg/dL) | 0.52 (0.49–1.43) | 0.52 | 1.33 (0.53–3.32) | 0.55 |
| CRP (mg/L) | 0.90 (0.63–1.29) | 0.56 | 0.88 (0.63–1.49) | 0.63 |
| Kt/Vb | 2.41 (0.08–74.84) | 0.62 | 0.46 (0.01–266.99) | 0.81 |
CI, confidence interval; CKD-aP, chronic kidney disease-associated pruritus; CRP, C-reactive protein; NB-UVB, narrowband ultraviolet B; HD, hemodialysis; PTH, parathyroid hormone; TSAT, transferrin saturation; 25(OH)D, 25-hydroxy vitamin D.
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,232 View
- 87 Download
- ORCID iDs
-
Youn Kyung Kee

https://orcid.org/0000-0002-0555-9909Hee Jung Jeon

https://orcid.org/0000-0003-3264-8525Jieun Oh

https://orcid.org/0000-0001-9429-9602Dong Ho Shin

https://orcid.org/0000-0002-6817-8716 - Related articles
-
Cinnamon: an aromatic condiment applicable to chronic kidney disease2023 January;42(1)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















