| Kidney Res Clin Pract > Volume 43(2); 2024 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This study was supported from the Shin Kong Wu Ho-Su Memorial Hospital Research Foundation (grant no. 2018SKHADR007).
Supplementary Materials
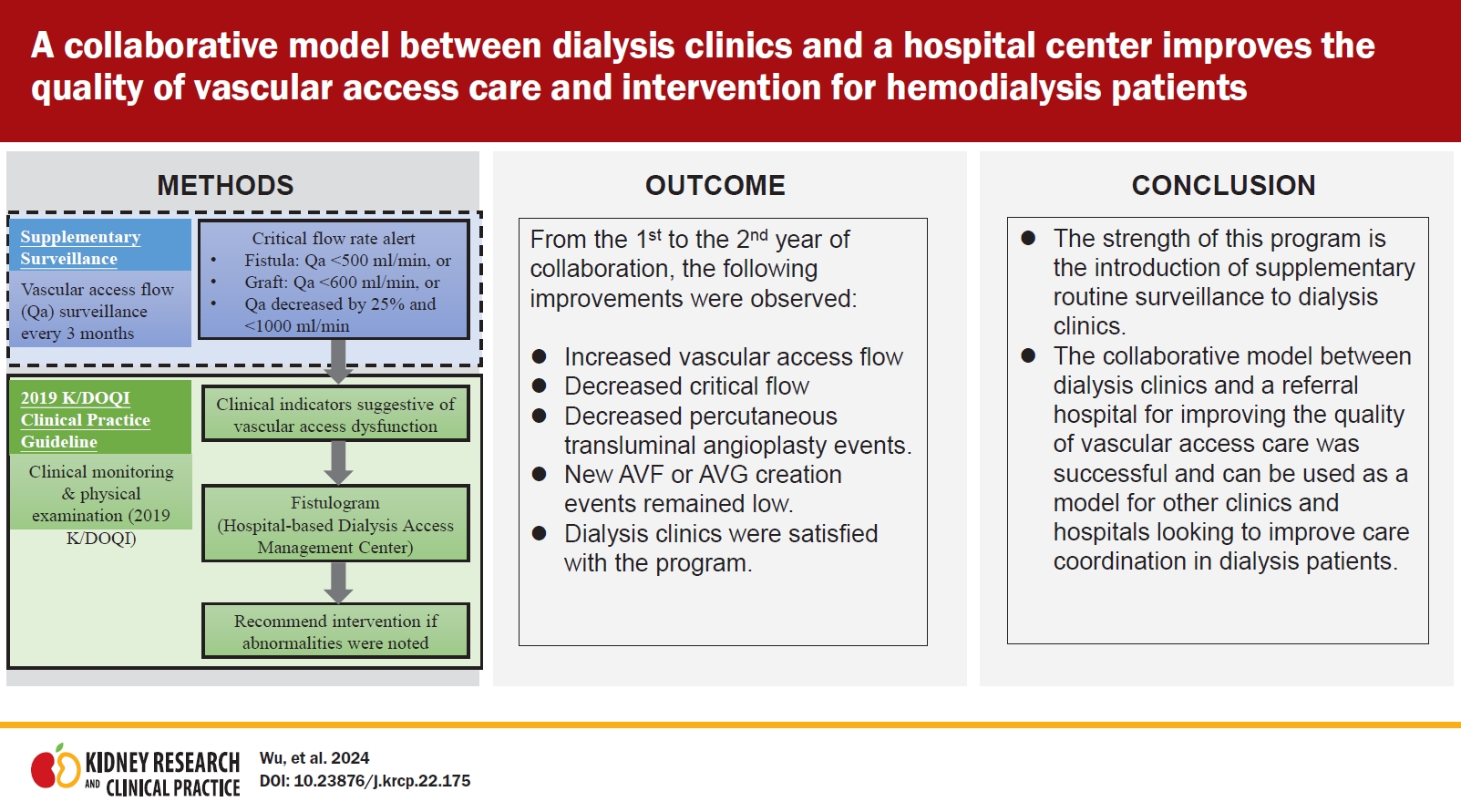
Figure 1.
Recommended management strategies of the collaborative program involving supplementary routine surveillance.
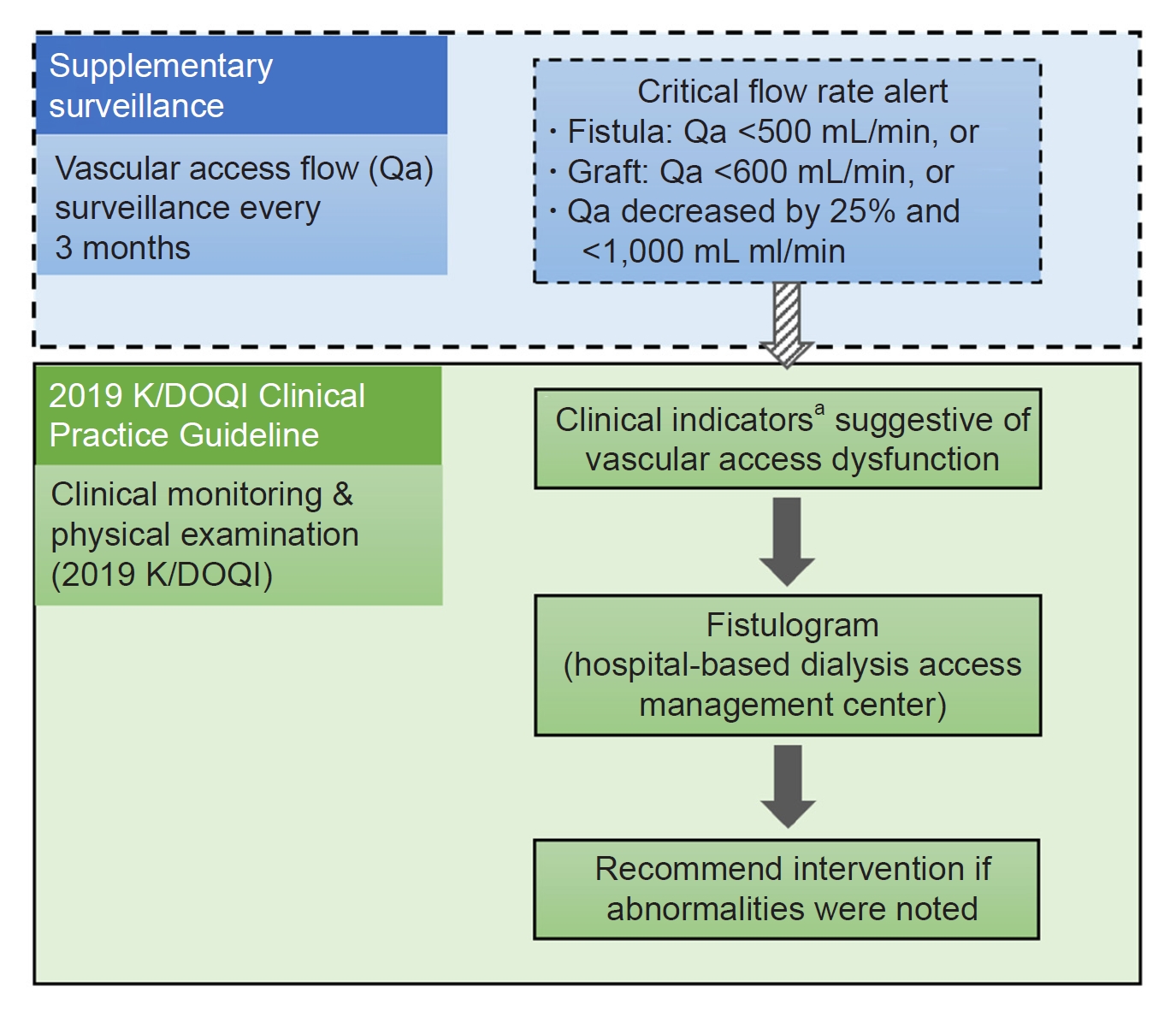
Figure 2.
Comparison of satisfaction survey responses from clinics between 2019 and 2020.

Figure 3.
Comparison of the referral rate and distance between hospitals and clinics between 2019 and 2020.
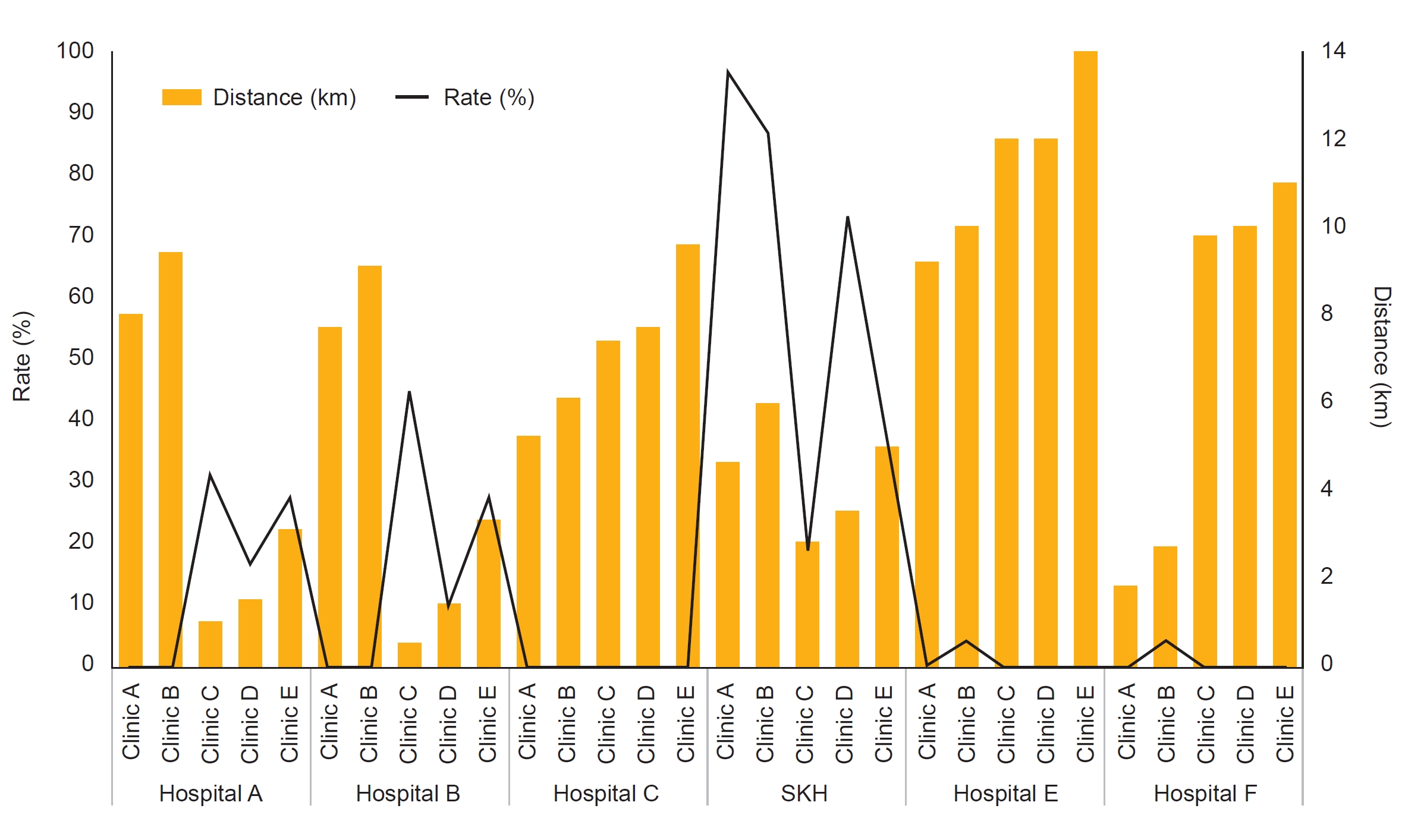
Table 1.
| Characteristic | AVF | AVG | p-value |
|---|---|---|---|
| No. of subjects | 339 | 53 | |
| Age (yr) | 65.0 (59.0–73.0) | 67.0 (61.0–77.0) | 0.20a |
| SBP (mmHg) | 140 (123–155) | 131 (118–146) | 0.03a,* |
| DBP (mmHg) | 70 (61–80) | 65 (59–75) | 0.03a,* |
| Sex | |||
| Female | 158 (46.6) | 38 (71.7) | 0.001b,* |
| Male | 181 (53.4) | 15 (28.3) |
Table 2.
| Variable |
AVF (N = 339) |
AVG (N = 53) |
||||
|---|---|---|---|---|---|---|
| 2019 | 2020 | p-value | 2019 | 2020 | p-value | |
| Ratio of critical flowa | ||||||
| Overall | 207/1,133 (18.3) | 123/972 (12.7) | 0.0004b,* | 44/168 (26.2) | 26/124 (21.0) | 0.30 |
| Clinic A | 72/460 (15.7) | 47/414 (11.4) | 0.06 | 17/68 (25.0) | 12/56 (21.4) | 0.64 |
| Clinic B | 23/129 (17.8) | 19/118 (16.1) | 0.72 | 3/17 (17.7) | 4/10 (40.0) | 0.20 |
| Clinic C | 60/277 (21.7) | 29/238 (12.2) | 0.005b,* | 14/36 (38.9) | 4/29 (13.8) | 0.02b,* |
| Clinic D | 18/116 (15.5) | 10/101 (9.9) | 0.22 | 1/8 (12.5) | 0/8 (0) | 0.30 |
| Clinic E | 34/151 (22.5) | 18/101 (17.8) | 0.37 | 9/39 (23.1) | 6/21 (28.6) | 0.64 |
| Vascular access flow | ||||||
| Overall | 1,035.80 ± 496.76 (n = 1,133) | 1,076.30 ± 513.65 (n = 972) | 0.07 | 972.38 ± 416.37 (n = 168) | 1,013.06 ± 391.44 (n = 124) | 0.40 |
| Clinic A | 1,101.43 ± 484.50 (n = 460) | 1,141.62 ± 545.87 (n = 414) | 0.25 | 998.53 ± 426.09 (n = 68) | 1,074.82 ± 445.51 (n = 56) | 0.33 |
| Clinic B | 1,063.64 ± 441.91 (n = 129) | 1,100.17 ± 507.73 (n = 118) | 0.55 | 956.47 ± 182.82 (n = 17) | 769.00 ± 223.68 (n = 10) | 0.03c,* |
| Clinic C | 972.38 ± 474.54 (n = 277) | 991.89 ± 423.28 (n = 238) | 0.63 | 778.06 ± 369.95 (n = 36) | 884.48 ± 229.71 (n = 29) | 0.16 |
| Clinic D | 1,172.76 ± 636.84 (n = 116) | 1,159.50 ± 579.97 (n = 101) | 0.87 | 1,413.75 ± 478.51 (n = 8) | 1,366.25 ± 390.93 (n = 8) | 0.83 |
| Clinic E | 823.18 ± 413.19 (n = 151) | 896.34 ± 440.00 (n = 101) | 0.18 | 1,022.56 ± 422.81 (n = 39) | 1,007.62 ± 363.70 (n = 21) | 0.89 |
| Clinical event ratio | ||||||
| No. of PTAs | 0.324 (n = 110) | 0.316 (n = 107) | 0.80 | 0.774 (n = 41) | 0.509 (n = 27) | 0.005b,* |
| No. of new AVF creations | 0.003 (n = 1) | 0 (n = 0) | 0.32 | 0 (n = 0) | 0.038 (n = 2) | 0.15 |
| No. of new AVG creations | 0.006 (n = 2) | 0 (n = 0) | 0.16 | 0.057 (n = 3) | 0 (n = 0) | 0.08 |
Data are expressed as number (%), mean ± standard deviation, or ratio only. Event ratio = number of critical flow events / number of records in 1 year.
AVF, arteriovenous fistula; AVG, arteriovenous graft; N, number of participants; n, number of records; PTA, percutaneous transluminal angioplasty.
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,464 View
- 78 Download
- ORCID iDs
-
Chung-Kuan Wu

https://orcid.org/0000-0003-4446-0167Yu-Wei Fang

https://orcid.org/0000-0002-1302-1885Chia-Hsun Lin

https://orcid.org/0000-0003-1041-0516 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















