De novo glomerulitis associated with graft-versus-host disease after allogeneic hematopoietic stem cell transplantation: A single-center experience
Article information
Abstract
Background
Nephrotic syndrome (NS) and proteinuria are uncommon, often unrecognized manifestations of graft-versus-host disease (GVHD) after hematopoietic stem cell transplantation (HSCT). Only a few isolated case reports and case series involving smaller number of patients who developed NS after HSCT have been published.
Methods
We reviewed the renal histopathological examination findings and clinical records of 15 patients who developed proteinuria after HSCT at Seoul and Yeouido St. Mary′s Hospital (Seoul, Korea). We also measured the anti-PLA2R antibodies (M-type phospholipase A2 receptor) in the serum samples from the seven patients at the time of renal biopsy.
Results
All patients had GVHD. The most common indication for biopsy was proteinuria (>1 g/day), with nine patients having nephrotic range proteinuria. The most common histopathological finding was membranous nephropathy (MN; n = 12). Other findings were membranoproliferative glomerulonephritis, C1q nephropathy, and diabetic nephropathy. Eleven patients were treated with immunosuppressive agents, and three patients were treated only with angiotensin II receptor blocker. The overall response rate, including complete remission (urinary protein level <0.3 g/day) and partial remission (urinary protein level = 0.31–3.4 g/day), was 73%. The mean follow-up period was 26 months, and none of the patients developed end-stage renal disease. All of the seven patients with MN had negative findings for anti-PLA2R antibodies, measured using an enzyme-linked immunosorbent assay kit.
Conclusion
In this study the findings of 15 renal biopsies were analyzed and to our knowledge this is the largest clinicopathological study of GVHD-related biopsy-proven nephropathy. Approximately 80% of the patients were MN and 73% responded either partially or completely to immunosuppressive treatment. Currently, there is an increase in the incidence of GVHD-mediated renal disease, and therefore, renal biopsy is essential for diagnosing the nephropathy and preventing the progression of renal disease.
Introduction
Hematopoietic stem cell transplantation (HSCT) is an effective therapy for patients who require potent chemotherapy drugs for the treatment of certain diseases such as acute leukemia. The development of new chemotherapeutic agents and better control of infection have improved the survival rate of patients who undergo HSCT; however, these measures have also introduced certain long- and short-term complications. Chronic graft-versus-host disease (cGVHD) is a common late complication of allogeneic HSCT that occurs in 60–80% of long-term survivors, and its incidence is currently increasing [1]. In animal models, the kidney was defined as a target organ of cGVHD, but the underlying mechanisms and pathogenesis of GVHD-related renal disease in people are unclear [2].
The recognized renal problems in HSCT recipients are drug toxicity, radiation nephritis, infection, thrombotic microangiopathy, and glomerulonephritis. The etiology and pathogenesis of proteinuria, including nephrotic syndrome (NS) in HSCT recipients remain unclear; however, an association between NS and cGVHD has been proposed. Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, and Cizman B [3] have reported that membranous nephropathy (MN) accounts for two-thirds and minimal change disease (MCD) accounts for one-third of the cases of NS in HSCT recipients. Renal biopsy is rarely performed after HSCT because of inappropriate general conditions and patients’ reluctance. Therefore, only a few reports of renal histopathological analysis after allogeneic HSCT are available in the literature, and these consist of only case reports or case series involving smaller number of patients.
In this study, we have analyzed the clinicopathological characteristics of 15 HSCT recipients having urinary protein levels >1 g/day. To our knowledge, this is the largest series assessing patients with biopsy-proven nephropathy after HSCT.
Methods
We reviewed the renal pathology records of the patients at Seoul and Yeouido St. Mary′s Hospital (Seoul, Korea) and identified 15 renal biopsy specimens from 15 patients obtained after allogeneic HSCT between January 2000 and January 2013. During that period, approximately 2,800 procedures of allogeneic HSCT were performed. The renal biopsy specimens were routinely divided into three portions for examination: (1) tissues fixed in 10% formalin and embedded in paraffin, after which 3-μm thick sections were cut and stained with hematoxylin and eosin, periodic acid-Schiff, silver methenamine, and Masson′s trichrome. The sections were viewed using light microscopy (LM); (2) the tissue section was fixed in glutaraldehyde and examined under an electron microscope (EM); and (3) the tissue was examined with direct immunofluorescence microscopy (IF) for the deposition of immunoglobulin G (IgG), IgA, IgM, C3, C1q, fibrinogen, and light chains. Data concerning the patient characteristics, clinical course, and outcome were collected from their medical records. Responses to treatment were categorized as complete remission (CR), partial remission (PR), or nonresponse. The CR and PR were defined as a reduction of urinary protein levels to <0.3 g/day and urinary protein levels between 0.31 g/day and 3.4 g/day, respectively [4]. We measured the anti-PLA2R (M-type phospholipase A2 receptor) antibodies using a commercial antigen-specific enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource Inc., San Diego, CA, USA) in the serum samples from seven patients at the time of renal biopsy.
Results
Patient characteristics and histopathological biopsy findings
Patient clinical features and histopathological findings of the renal biopsies are summarized in Table 1. The incidence of biopsy-proven renal disease, including NS, was approximately 0.5% (15 cases among a total of 2,800 allogeneic HSCT recipients). A total of 15 renal biopsies from 15 patients who had previously undergone allogeneic HSCT were examined. The mean age of the patients was 39 years (range: 21–56 years), and nine of the 15 patients were male. All patients had normal renal function prior to the HSCT and absence of proteinuria. Cases 3, 14, and 15 had a history of diabetes mellitus without proteinuria and Case 14 had chronic hepatitis B and they all received lamivudine prior to undergoing HSCT. The mean duration between HSCT and renal biopsy was 40 months (range: 8–144 months). The underlying diseases resulting in the need for HSCT were acute lymphoid leukemia, six patients; acute myeloid leukemia, five patients; chronic myeloid leukemia, two patients; aplastic anemia, one patient; and myelodysplastic syndrome, one patient (Fig. 1). cGVHD was defined as pathological findings when it appeared 100 days after allogeneic HSCT. All cGVHD events were preceding the onset of proteinuria. Six of the 15 patients were taking immunosuppressive agents at the time of kidney biopsy (Table 2). The most common indication for biopsy was proteinuria, which was present in all patients; the urinary protein levels were within the nephrotic range in nine patients (Fig. 1).

Clinical and histopathological findings of the 15 patients with graft-versus-host disease after hematopoietic stem cell transplantation
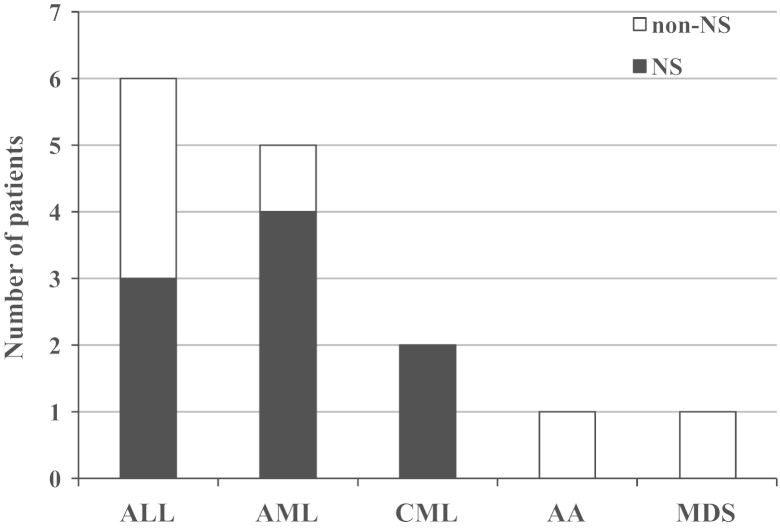
The underlying hematologic diseases in hematopoietic stem cell transplantation patients who received renal biopsies for either nephrotic syndrome (NS) or non-nephrotic range proteinuria. AA, aplastic anemia; ALL, acute lymphoid leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; MDS, myelodysplastic syndrome.
Renal histopathological findings
The most common histopathological finding was MN (n = 12). Other glomerular diseases identified were membranoproliferative glomerulonephritis (MPGN), C1q nephropathy, and diabetic nephropathy. Among the patients who had MN, seven had full-blown NS. Biopsy specimens from the MN patients indicated typical pathological features of the disease including thickening of basement membrane on LM (Fig. 2A) and fine granular deposit of IgG and C3 on IF; moreover, EM indicated the presence of electron-dense, subepithelial immune deposition (Fig. 2B). Renal biopsies from these patients did not demonstrate interstitial fibrosis with tubular atrophy and inflammatory cell infiltration.

Renal biopsy findings from a patient with membranous nephropathy. (A) A light micrograph illustrating preserved glomerular architecture, but thickened capillary walls (Periodic acid-Schiff stain 400X). (B) An electron micrograph illustrating electron-dense subepithelial deposits and effacement of epithelial cell foot processes (6,000X).
The renal biopsy specimen of a patient (Case 13) indicated diffuse global capillary wall thickening and mesangial expansion on LM and subendothelial and mesangial electron-dense deposit on EM. The IF indicated IgG and IgM deposition in the glomerular basement membrane (GBM) and mesangium. Thus, the patient was diagnosed with type 1 MPGN (Table 1). The renal biopsy specimen of another patient (Case 14) indicated global glomerular sclerosis in 50% of glomeruli and moderate mesangial expansion. Because this patient had type 2 diabetes, the diagnosis of diabetic nephropathy was made. The renal biopsy specimen of another patient (Case 15) indicated mesangial cell proliferation associated with mesangial deposits on EM and C1q deposition on IF; these findings were compatible with the diagnosis of C1q nephropathy.
Results of anti-PLA2R antibody measured using ELISA kit
The serum samples of seven patients with MN were collected at the time of renal biopsy and their anti-PLA2R titers were measured. All study patients had negative findings for anti-PLA2R antibodies.
Response to treatment and clinical course
The patient responses to treatment, determined based on follow-up tests of renal functions [serum creatinine and estimated glomerular filtration rate (GFR) levels, which were calculated using the Modification of Diet in Renal Disease formula (MDRD estimated GFR)], is summarized in Table 2. End-stage renal disease (ESRD) did not develop in any patient. Nine patients with MN were treated with prednisolone (2 mg/kg) that was administered on alternative days. Two patients with MN did not respond to prednisolone therapy, and therefore, additional treatment involving cyclosporine was initiated. A patient (Case 2), who had been previously treated with azathioprine developed bronchiectasis and recurrent infections associated with pulmonary GVHD; therefore, this patient was not treated with other immunosuppressive agents. However, we lost this patient to follow-up and was thus considered a nonresponder. Another patient (Case 9), who was receiving cyclosporine prior to being diagnosed with MN, was treated with angiotensin II receptor blocker (ARB) and achieved PR. Moreover, a patient (Case 12) who was treated with only ARB achieved PR. Among the 12 patients with MN, the CR rate was 25% and the PR rate was 58%. In a patient (Case 13) with MPGN type 1, initial treatment with prednisolone was started, followed by administration of low-dose prednisolone and azathioprine. This patient achieved PR. A patient (Case 14) with diabetic nephropathy was referred to a local clinic for further treatment, whereas another patient (Case 15) with C1q nephropathy died from sepsis due to complications of GVHD 2 weeks after undergoing renal biopsy.
Discussion
The outcomes of HSCT recipients have improved due to the development of novel chemotherapeutic agents, effective prevention, and treatment of infections, and new transplant regimens, such as nonmyeloablative transplantation. However, this improvement has been accompanied by an increase in glomerular or interstitial renal disease.
All our patients exhibited evidence of acute GVHD or cGVHD, which was present prior to or simultaneously with the renal disease. The GVHD frequently affects the skin, oral mucosa, liver, and gastrointestinal tract. The pathophysiology of GVHD is not well understood. In a murine model of cGVHD, renal involvement of cGVHD has been commonly detected; however, renal involvement of cGVHD in humans has been rarely noted [5]. Inflammatory and cytokine cascade, such as those involving tumor necrosis factor-α and/or interferon-γ, from donor T cell may be crucial for the development of the renal disease in cases with GVHD [6]. Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, and Cizman B [3] have proposed that withdrawal or reduction in the dose of immunosuppressive agents in GVHD treatment is a risk factor for the development of NS.
Among the different types of glomerulopathies, MN is reportedly the most common, followed by MCD [7], which is consistent with the findings of this study as well (80%). The overall response rate (CR and PR) was 73%. At present, there are no standard treatment guidelines for NS after HSCT. Niscola P, Tendas A, Luo XD, Catalano G, Scaramucci L, Cupelli L, Giovannini M, Ferranini M, Bondanini F, Piccioni D, Dentamaro T, Palumbo R, Perrotti AP, Liu QF, and de Fabritiis P [7] analyzed 69 previously reported cases of MN and found that the treatments used were very highly variable, the most frequently used agent was corticosteroid followed by cyclosporine. In that study, 59% patients achieved CR and 28% achieved PR. Treatment failed in the remaining 13% of patients, and ESRD developed in a few patients [7]. In this study, the combined response rate of CR and PR in patients with MN was 73%, which is similar to that previously reported [7]. Approximately 30–35% of untreated patients with idiopathic MN have benign course of the disease or spontaneous resolution, and the decision to initiate treatment using immunosuppressive agent should be made based on the patients’ risk factor such as proteinuria levels or deteriorating of renal function [4]. However, in patients with secondary MN, the treatment of the underlying disease induced a remission of MN (e.g., the treatment of neoplasm in case of malignancy-associated MN). Therefore, we initiated the immunosuppressive agent treatment in all the patients with MN, in order to treat the renal GVHD.
MN is the most common form of immune-complex-mediated glomerulonephritis reported in association with HSCT [6], [7], [8]. MN is caused by the deposition of immune complexes in the subepithelial zone of glomerular capillaries, but its underlying pathogenic mechanism is not completely understood. The role of anti-PLA2R (M-type phospholipase A2 receptor) antibodies in primary MN has recently been elucidated [9]. Although anti-PLA2R antibody was detected in approximately 70% of patients with primary MN, some patients with secondary MN also exhibited the antibody. Moreover, Qin W, Beck LH Jr, Zeng C, Chen Z, Li S, Zuo K, Salant DJ, and Liu Z. [10] detected anti-PLA2R antibody in 6.3% of patients with hepatitis B virus-associated MN and in 30% of patients with tumor-associated MN. By contrast, the etiology and significance of anti-PLA2R antibody in secondary MN are not known, especially in HSCT with GVHD. Moreover, the relationship between serum concentration of the antibody and the clinical course of MN is unknown and requires additional investigation. In our study, seven patients whose serum samples were collected at the time of renal biopsy had negative results for the anti-PLA2R antibodies in the ELISA test. This result suggests that MN associated with GVHD has a different pathogenesis from idiopathic MN. It has been well known that the antibody directed against tubular brush-border antigen induced tubulointerstitial fibrosis and tubular atrophy in a murine GVHD model [11]. However, our patients with MN did not show a prominent tubular injury, because they were in the early phase of MN.
In addition to MN, the other common glomerulopathy was MCD. MCD accounts for approximately one-quarter of the cases of NS after HSCT [3], [12]. The treatment response of patients with MCD is better than that of MN patients, with a previous study reporting that 90% of its study patients had achieved CR [3]. In this study, however, none of the patients had MCD. One of our patients had MPGN type 1 and was successfully treated with prednisolone and azathioprine. In addition, a patient with C1q nephropathy died from GVHD-associated sepsis.
Although the mechanisms underlying the development of NS or proteinuria associated with GVHD have not been elucidated, it has been hypothesized to represent the end stage of alloreactivity, in which T cells have evolved to assume the Th2 phenotype [13], [14]. A proposed mechanism for this evolution is immune dysregulation resulting from the transfer of alloreactive peripheral donor lymphocytes (possibly, CD8+/perforin+ cytotoxic T cells) present in the primary blood stem cell graft, with reactivity toward glomerular antigens [15]. The CD8+/perforin+ cytotoxic T lymphocytes might induce the destruction of glomeruli and apoptosis of podocytes and endothelial cells, producing microthrombi, and finally destroying the loop segments [15]. Another hypothesis is that the induction of GVHD in rats may lead to restricted polyclonal stimulation of B cells and the formation of autoantibodies directly targeting basement membrane. Consequently, immunoglobulin deposition along the GBM and development of proteinuria were found [11].
In summary, we have analyzed the clinicopathological features of 15 patients who have developed proteinuria or NS in association with GVHD after allogeneic HSCT. The most common histopathological renal finding was MN and most patients with MN responded well to immunosuppressive agents. Because the population of long-term survivors after allogeneic HSCT is growing, the incidence of GVHD-mediated renal disease may increase. Therefore, renal biopsy is essential in establishing the cause of the renal dysfunction and in directing the proper immunosuppressive therapy.
Conflicts of interest
We have no conflicts of interest to declare.
References
Acknowledgments
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health and Welfare, Republic of Korea (C.W.P.; Grant No. A111055).
