The effect of on-line hemodiafiltration on heart rate variability in end-stage renal disease
Article information
Abstract
Background
The autonomic nervous system plays a central role in the maintenance of hemodynamic stability. Cardiac autonomic dysfunction may result in serious complications, such as sudden cardiac death. Heart rate variability (HRV) is significantly reduced in patients undergoing chronic hemodialysis (HD). The aim of this study was to evaluate the effect of on-line hemodiafiltration (OL-HDF) on the autonomic nervous system in chronic HD patients.
Methods
Forty chronic HD patients were prospectively studied. The participants were divided into conventional HD and OL-HDF groups. They received regular high-flux HD or OL-HDF for 4-hour sessions, three times a week. Time-and frequency-domain measures of the 24-hour HRV were analyzed during the interdialytic period prior to postdilution OL-HDF and every 6 months for 24 months. The 7-year survival was also evaluated.
Results
Among the 40 participants, 15 patients in the HD group and 11 patients in the OL-HDF group completed the study. There was no difference in the baseline characteristics. After 24 months of treatment, β2-microglobulin concentration decreased (from 33.4±15.2 mg/dL to 28.4±6.2 mg/dL, P =0.02) in the OL-HDF group, while there was no change in the HD group. In the HRV analysis, the frequency-domain HRV parameters increased significantly compared with baseline in the OL-HDF group [natural logarithmic high frequency (lnHF), 3.15±3.36 ms2 vs. 4.42±3.81 ms2; ln low frequency (LF), 3.56±3.17 ms2 vs. 4.78±3.99 ms2; ln very low frequency (VLF), 4.90±4.62 ms2 vs. 6.38±5.54 ms2; LF/HF ratio, 1.4±0.4 vs. 2.5±0.1]. The survival rate was similar between the groups.
Conclusion
This study shows that OL-HDF improved autonomic nervous system dysfunction in chronic HD patients.
Introduction
The autonomic nervous system plays a central role in the maintenance of hemodynamic stability. Cardiac autonomic dysfunction may result in serious complications, such as sudden cardiac death.
Spectral analysis of heart rate variability (HRV) has emerged in the past decade as a powerful noninvasive clinical tool for the assessment of sympathetic and parasympathetic functions of the autonomic nervous system [1]. Rubinger et al [2] reported that HRV is significantly reduced in patients undergoing chronic hemodialysis (HD), even in the absence of cardiovascular disease. Decreases in some HRV measures were suggested to be independent predictors of cardiac death during long-term follow-up in patients with end-stage renal disease receiving chronic HD [3].
On-line hemodiafiltration (OL-HDF) was introduced as a model for the effective clearance of middle-to-large molecules [4]. Compared with standard HD, OL-HDF improves hemodynamic stability and reduces mortality [5], [6].
In a study with 32 dialysis patients, Laaksonen et al [7] reported that higher Kt/V (>1.20) was a predictor of improvement of cardiac autonomic nervous function.
The aim of this study was to evaluate the effect of OL-HDF on the autonomic nervous system in chronic HD patients. We hypothesized that OL-HDF improves the autonomic neuropathy in these patients.
Methods
Patients
Forty outpatients with end-stage renal disease were recruited who received regular chronic HD therapy for at least 3 months (4-hour sessions, three times a week, high-flux hemodialysis) at the hemodialysis room of Kwandong University Myongji Hospital between 2005 and 2006. The participants were randomized into conventional HD and OL-HDF groups. Patients with a history of any of the following medical events were excluded at baseline: myocardial infarction, stroke, a major surgical procedure within the previous 2 months, ≥NYHA 3 congestive heart failure, hemodynamically significant valvular or congenital heart disease, atrial fibrillation or flutter, high grade heart block or a permanent pacemaker, chronic obstructive lung disease, severe hepatic disease, malignant neoplasms, or other physical or mental problems that limit normal daily activities. The study followed the Helsinki Declaration and Good Clinical Practices.
OL-HDF technique
OL-HDF patients received postdilution OL-HDF for 4 hours, three times weekly for 24 months with bicarbonate dialysis fluid and heparin as an anticoagulant. OL-HDF was performed using the AK200 ULTRA S (Gambro, Lund, Sweden) with nonreprocessed polyamide dialysis membranes (Polyflux 14; Gambro). Blood flow was maintained at least 250 mL/minute, the dialysate flow was 600 mL/minute, and the temperature of the dialysate was approximately 36 °C.
Laboratory methods
Blood samples were drawn every 6 months for routine laboratory assessments including hemoglobin, blood urea nitrogen, creatinine, calcium, phosphorus, albumin, total cholesterol, triglyceride, uric acid, high sensitivity C-reactive protein, and β2-microglobulin (β2-MG). The Kt/V was calculated every 6 months for 2 years.
HRV analysis
Holter electrocardiogram (ECG) monitoring for 24 hours for power spectral analysis of the RR intervals was performed in the inter-dialytic period every 6 months for 24 months. Recorded Holter ECGs were analyzed using a Holter ECG scanner (Marquette ECG Analysis Program; GE Medical Systems, Waukesha, WI, USA), which automatically detected and labeled the QRS complexes. The results of the automatic analysis were reviewed, and errors in the R-wave detection and the QRS labeling were edited manually.
For the time-domain HRV measures, the mean normal-to-normal R-R intervals (NN) and the standard deviation of the normal-to-normal R-R intervals over the 24 hours (SDNN) measurements were calculated.
To analyze the frequency-domain HRV measures, the spectral power was quantified using fast Fourier transformation for the following frequency bands: 0.15–0.40 Hz (high frequency), 0.04–0.15 Hz (low frequency), 0.003–0.04 Hz (very-low frequency), and ≤0.003 Hz (ultra-low frequency). The variances were transformed to natural logarithmic (ln) values.
Outcome measures
The primary outcome was improvement of autonomic neuropathy assessed with HRV, and the secondary outcome was 7-year mortality. The patients were followed for mortality analysis until December 2012 or until they received a kidney transplant. We regarded transplants as censored cases. For patients who moved to other facilities, we recruited the mortality data by telephone.
Statistical analysis
Continuous data are reported as the mean±standard deviation. Categorical data are presented as absolute values and percentages. The differences between the two groups were tested using the Fisher exact or the Chi-square test for categorical variables and the Student t test or Mann–Whitney test for continuous data. The serial HRV measures were compared within and between the groups using repeated-measures analysis of variance. We performed a survival analysis using the Kaplan–Meier survival curve. All the statistical analyses were performed using SPSS software version 19.0 (IBM, Chicago, IL, USA). A 2-tailed P<0.05 was considered to be statistically significant.
Results
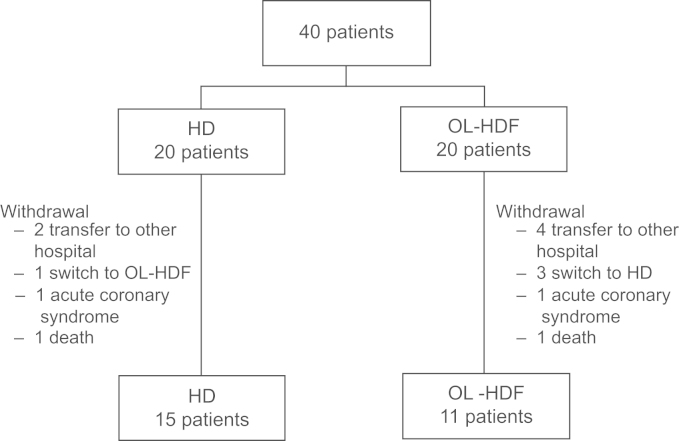
Among the 40 patients, 15 patients in the HD group and 11 patients in the OL-HDF group completed the entire study (Fig. 1). Two patients died during the study period: one in the HD group from sepsis, and one in the OL-HDF group from gastric ulcer bleeding. In total, 26 patients were included in the study. The baseline characteristics are compared in Table 1. The HD and OL-HDF groups showed similar characteristics. The median age of the patients was 61 years in both groups (ranges, 49–70 years in HD group and 36–80 years in OL-HDF group). The mean dialysis vintages were 38.6 months (range, 3–125 months) and 35.3 months (range, 3–160 months) in the HD and OL-HDF groups, respectively.
The baseline laboratory findings including hemoglobin, calcium, phosphorus, blood urea nitrogen, creatinine, albumin, electrolytes, parathyroid hormone, C-reactive protein, β2-MG, and Kt/V were not different between the HD and OL-HDF groups (Table 2).
During the 24-month follow up, β2-MG concentration exhibited a nonsignificant increase in the HD group (34.6±16.3 mg/dL to 37.3±9.1 mg/dL, P=0.060) and a significant decrease in the OL-HDF group (33.4±15.2 mg/dL to 28.4±6.2 mg/dL, P=0.013).
Changes of HRV parameters
Table 3 shows the HRV parameters. The baseline HRV measures were not different between the HD and OL-HDF groups. There was no difference in the time-domain measures during the follow-up period between the HD and OL-HDF groups except for low SDNN in the HD group and high mean NN in the OL-HDF group at 24 months. The frequency-domain measures were obviously different between the two groups. The frequency-domain measures did not differ between the two groups at baseline but increased continuously over time in the OL-HDF group (Fig. 2).
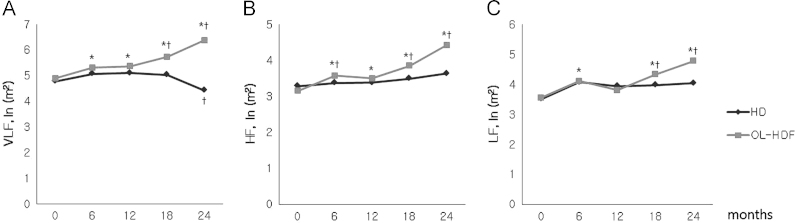
Changes of the heart rate variability measurement. The frequency domain parameters [(A) very low frequency, VLF; (B) high frequency, HF; (C) low frequency, LF] increased during on-line hemodiafiltration (OL-HDF) treatment. ⁎P<0.05 vs. hemodialysis, †P<0.05 vs. baseline. NN, normal-to-normal R-R intervals; SDNN, standard deviation of normal-to-normal R-R intervals during 24 h.
Survival analysis
Five patients in the HD group and four patients in the OL-HDF group transferred to other facilities after the study completion. Among patients who stayed at our hospital, seven in the HD group and four in the OL-HDF group maintained their HD modality without change.
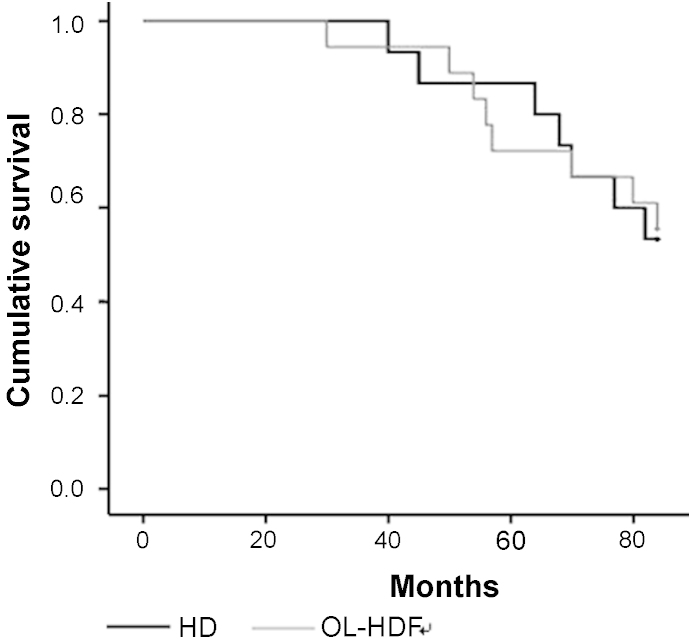
Five patients in the HD group (45.5%) and seven patients in the OL-HDF group (46.7%) died during the follow-up period. The Kaplan–Meier survival curve showed that the cumulative survival was not different between the groups (Fig. 3).
Discussion
The major finding of our study was that OL-HDF improved autonomic nervous system dysfunction measured by HRV in chronic HD patients.
Many HD patients have autonomic neuropathy. Ewing DJ and Winney R [8] found that half of the HD patients exhibited autonomic neuropathy using three simple methods including responses to the Valsalva maneuver, handgrip response, and beat-to-beat variation of the heart beat at rest. Several studies have reported that hypotension during HD may be caused by autonomic neuropathy [9], [10], [11]. Many of HD patients without intradialytic hypotension also experience autonomic neuropathy symptoms including impotence, postural dizziness, and uremic pruritus [12], [13], [14].
Analysis of HRV has been used as a noninvasive tool to assess the autonomic nervous system. Although there are various ways of quantifying HRV, including time domain, frequency domain, and nonlinear analysis, one method has not been established as superior to another [15], [16], [17]. Recently, Suzuki et al [18] reported that nonlinear measures of HRV had predictive value for mortality in HD patients. We analyzed HRV conventionally using 24-hour Holter ECGs for time and frequency domain analyses according to the guidelines [19].
Uremic cardiac autonomic neuropathy may be characterized by reduced HRV indicating cardiac sympathetic overactivity and parasympathetic deterioration [20]. Several studies have reported reduced HRV [21] and its association with left ventricular hypertrophy [22] and increased CKD-related hospitalization [23].
The role of dialysis in autonomic neuropathy has been reported by several investigators. Leem et al [24] reported that dialysis did not alter autonomic nerve function during the first 12 months of HD. However, Laaksonen et al [7] found that the improvement in HRV occurred only in patients who had a Kt/V>1.2. Progressive deterioration of autonomic neuropathy was associated with a Kt/V<0.87. In addition, Dursun et al [25] suggested that dialysis for 12 months improved autonomic dysfunction, especially in continuous ambulatory peritoneal dialysis (CAPD).
Genovesi et al [26] suggested a potential autonomic advantage with convective treatment (hemofiltration) compared with diffusive treatment (hemodialysis) during both interdialytic and intradialytic periods using spectral analysis of HRV. The role of convection in treatment of autonomic neuropathy is not fully understood. Santoro et al [27] suggested that hemofiltration acts through less negative sodium balance and effective removal of vasodilating or autonomic nervous inhibitory substances. In the present study, OL-HDF for 24 months improved autonomic neuropathy measured by HRV analysis. It is well known that many patients with systemic amyloidosis have autonomic neuropathy and they show abnormal HRV [28], [29]. Most uremic patients have high β2-MG concentration and this causes uremic amyloidosis. In our study, OL-HDF effectively removed β2-MG with the convective method; however, we cannot find any causal relationship between β2-MG and autonomic neuropathy. Rubinger et al [2] reported that renal transplantation normalized HRV in most uremic patients except the amyloidosis patients with cardiac or adrenal involvement. However, they did not reveal the relationship between β2-MG and HRV.
In contrast to previous studies, we failed to show improved survival in the patients treated with OL-HDF [6], [30]. The small study population and the inability of some patients to maintain their HD modality after moving to other hospitals might be barriers to confirming improved survival.
Our study has several limitations. First, the sample size was small and the study was performed at a single center. It is well known that the majority of HD patients are diabetics and abnormal HRV is very common in patients with diabetes regardless of renal function [31], [32]. Unfortunately, because of the small sample size, we could not compare the effect of OL-HDF on HRV in diabetics with nondiabetics. Second, we recorded Holter electrocardiography during the interdialytic period, which may not incorporate intradialytic arrhythmia. Third, we were unable to obtain information concerning the HD modality, (i.e., HD vs. OL-HDF) in the patients who were transferred to other facilities.
To our knowledge, this is the first prospective study to examine the effect of OL-HDF on autonomic neuropathy in chronic HD patients. Large-scale studies are required to confirm the role of OL-HDF in the improvement of autonomic neuropathy in HD patients.
Conflicts of interest
The authors have no conflicts of interest to declare.
References
Acknowledgments
We thank Ms. Mira Yoon for her technical assistance in the HRV analysis.