| Kidney Res Clin Pract > Volume 32(3); 2013 > Article |
|
Abstract
Idiopathic membranous nephropathy is a common cause of nephrotic syndrome, and has been reported as a cause of idiopathic primary glomerulonephropathy in up to 90% of patients. However, the treatment options remain controversial. We report two cases of idiopathic membranous nephropathy that were treated with rituximab. A 54-year-old man and a 64-year old man were admitted for rituximab therapy. They had previously been treated with combinations of immunosuppressive agents including cyclophosphamide, cyclosporine, mycophenolate, and steroids. However, the patients' heavy proteinuria was not resolved. Both patients received rituximab therapy, 2 weeks apart. After several months of follow-up and a second round of rituximab treatment for each patient, their proteinuria decreased and partial remission of disease was achieved in both patients.
Keywords
Idiopathic membranous nephropathy, Nephrotic syndrome, Proteinuria, RituximabMembranous nephropathy is a common cause of nephrotic syndrome in adults [1]. With a relatively slow disease progression, membranous nephropathy progresses to end-stage renal disease in approximately 40% of patients [2]. Currently, treatment includes corticosteroids, alkylating agents, cyclosporine, mycophenolate mofetil, and tacrolimus [1]. However, drug toxicity and persistent heavy proteinuria resistant to these drugs are problematic in many patients. In rodent models, B cells have been implicated in the pathogenesis of idiopathic membranous nephropathy [3]. Therefore, rituximab, a selective B cell targeting agent, has emerged as an alternative treatment option for membranous nephropathy. Several studies evaluating the effectiveness of rituximab therapy for membranous nephropathy have shown promising results [2], [4], [5]. However, the treatment of idiopathic membranous nephropathy with rituximab has not been reported in Korea. We report two cases of membranous nephropathy showing partial remission after rituximab treatment.
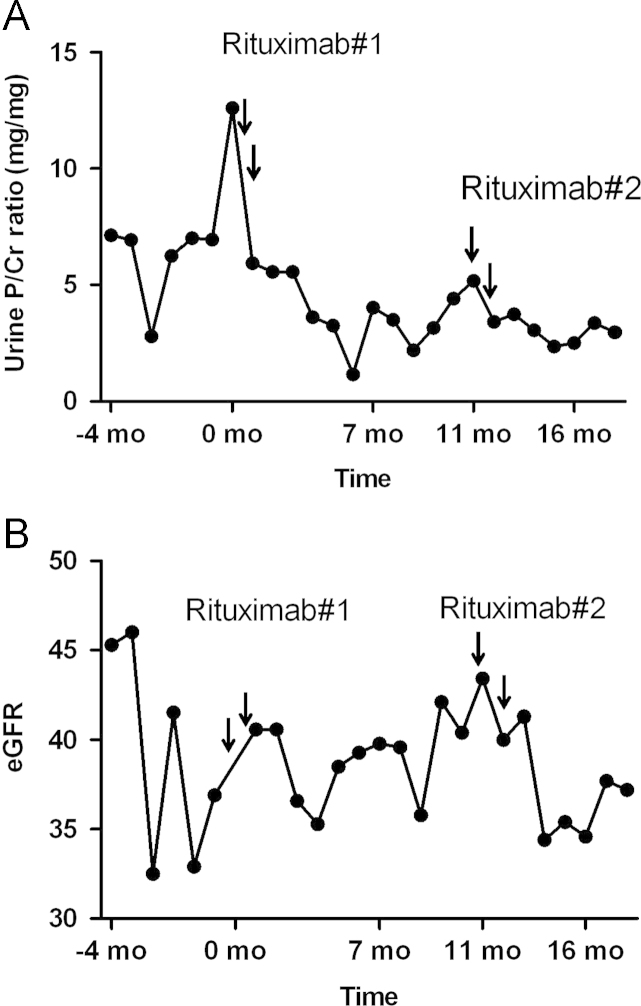
A 64-year-old man with a 21-year history of idiopathic membranous nephropathy presented with increased proteinuria. He had been taking angiotensin-converting enzyme inhibitors and angiotensin receptor blockers with no response. Mycophenolate 1,000┬Āmg/day and prednisolone 30┬Āmg/day were administered for 1 year, followed by cyclophosphamide 100┬Āmg/day and prednisolone 5┬Āmg/day for 6 months; both regimens were unsuccessful for resolving his proteinuria. Cyclosporine 200┬Āmg/day and prednisolone 5┬Āmg/day were subsequently prescribed for 5 years, with a temporary decrease in proteinuria for 2 years to 2┬Āg/day; however, proteinuria gradually increased to 5┬Āg/day. Mycophenolate 1,000┬Āmg/day was then added to the cyclosporine and prednisolone regimen. However, azotemia exacerbation, serum creatinine level of 1.92┬Āmg/dL, and persistent heavy proteinuria were noted. Despite these immunosuppressive treatments, the patient's proteinuria increased to 12┬Āg/day and his edema was aggravated. To exclude the possibility of other renal diseases, a renal biopsy was performed on July 30, 2010. A complete blood count at the time of the renal biopsy showed normocytic and normochromic anemia (hemoglobin=11.3┬Āg/dL), with a normal white blood cell and platelet count. Liver function tests were normal. Blood urea nitrogen (BUN) and creatinine levels were 23.7┬Āmg/dL and 1.86┬Āmg/dL, respectively. Renal biopsy showed stage III/IV membranous nephropathy. Because previous immunosuppressant regimens were ineffective, rituximab 1┬Āg was administered on Day 1 and Day 15. Valsartan 80┬Āmg was continued irrespective of rituximab. The patient did not experience any side effects during and after the infusion. Before rituximab infusion, the number of CD19(+) B cells was 425/╬╝L. Two months and 5 months after rituximab therapy, urine protein/creatinine ratios decreased to 5.55┬Āmg/mg and 1.14┬Āmg/mg, respectively. However, the patient's proteinuria increased slowly thereafter. After 6 months, the number of CD19(+) B cells decreased to 37/╬╝L, but it rose to 148/╬╝L at 8 months. After 10 months, the urine protein/creatinine ratio increased to 5.17┬Āmg/mg. With the increase of proteinuria and edema, the second round of rituximab treatment was performed according to the same dosage and schedule as the first treatment. Six months later, the patient's urine protein/creatinine ratio decreased to 2.95┬Āmg/mg and the number of CD19(+) B cells fell to 14/╬╝L (Fig. 1A). However, the estimated glomerular filtration rate (GFR) was declined (Fig. 1B). These results indicate that rituximab could be used for the decrement of proteinuria.
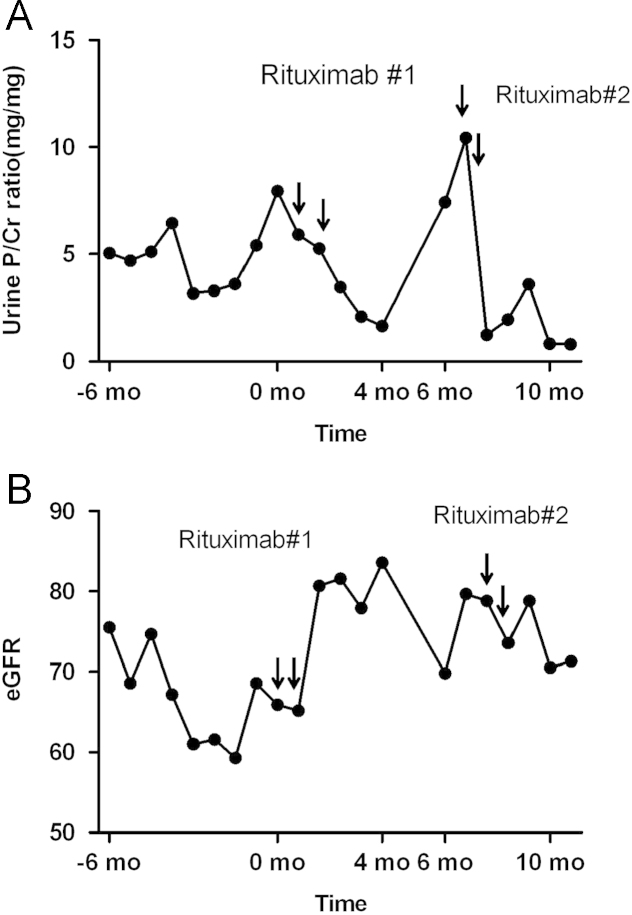
A 54-year-old man presented with generalized edema and foamy urine. He had been diagnosed with stage II/IV membranous nephropathy about 2 years prior. The patient was initially treated with angiotensin-converting enzyme inhibitor and angiotensin receptor blocker. However, nephrotic-range proteinuria persisted at approximately 6ŌĆō7┬Āg/day after 6 months. Cyclophosphamide 125┬Āmg/day and prednisolone 10┬Āmg/day were then administered for 5 months, with no response. Mycophenolate 2,000┬Āmg/day and prednisolone 10┬Āmg/day were subsequently administered for 5 months; the patient's proteinuria temporarily decreased to 0.3┬Āg/day, but soon increased to 5.0┬Āg/day. Mycophenolate was replaced by cyclosporine 200┬Āmg/day, but with no response. Finally, combination treatment of mycophenolate 2,000┬Āmg/day and cyclosporine 150┬Āmg/day was tried, but this regimen failed to elicit even partial remission. Because these treatment regimens proved unsuccessful, the patient was admitted for rituximab treatment. Complete blood count indicated normocytic and normochromic anemia (hemoglobin=10.7┬Āg/dL), with normal white blood cell and platelet counts. Total protein was 4.4┬Āg/dL, albumin was 2.3┬Āg/dL, and liver function tests were normal. BUN and creatinine levels were 34.6┬Āmg/dL and 1.17┬Āmg/dL, respectively. Spot urine protein/creatinine ratio was 7.95┬Āmg/mg. Rituximab 1┬Āg was administered on Day 1 and Day 15. Valsartan 80┬Āmg/day was continued irrespective of treatment. One month after the initiation of rituximab treatment, the patient's urine protein/creatinine ratio decreased to 3.45┬Āmg/mg and generalized edema improved. After 4 months, the patient's urine protein/creatinine ratio decreased further to 1.66┬Āmg/mg, and the number of CD19(+) B cells was 11/╬╝L. After 6 months, however, the urine protein/creatinine ratio increased to 7.42┬Āmg/mg, and a second round of rituximab treatment was performed. Five months later, the patient's urine protein/creatinine ratio fell to 0.80┬Āmg/mg (Fig. 2A). The estimated GFR improved 17% after rituximab therapy (Fig. 2B).
These two cases presented here demonstrate the potential of rituximab treatment for idiopathic membranous nephropathy in patients who do not respond to usual immunosuppressive treatment. During 1 year and 2 years of follow-up, the urine protein/creatinine ratios in our cases decreased after rituximab treatment. However, approximately 6 months following the initial treatment, rituximab was again required, and complete remission was not achieved.
Current treatments for idiopathic membranous nephropathy include steroids and immunosuppressant drugs, which are not disease-specific and are associated with serious risks of toxicity in the reninŌĆōangiotensin system blockade in nonresponders [2]. Two types of immunosuppressants are typically used in combination with corticosteroids: alkylating agents (cyclophosphamide and chlorambucil) and calcineurin inhibitors (cyclosporine or tacrolimus) [1]. Despite these powerful immunomodulating drug combinations, refractory membranous nephropathy cases have been reported. For these refractory membranous nephropathies, other management strategies have been tried with mycophenolate mofetil, tacrolimus, and intravenous immunoglobulin [6].
According to the Heymann nephritis model applied in rodent studies, B cells play an important role in the pathogenesis of idiopathic membranous nephropathy [7], [8]. Recently, several podocyte antigens, including neutral endopeptidase, M-type phospholipase A2 receptor, aldose reductase, and superoxide dismutase 2, were identified as targets for autoantibodies in patients with membranous nephropathy [9]. Rituximab, a chimeric antibody against B cell CD20 antigen, produces specific depletion of these cells for approximately 12 months [10]. Treatment for this antibody-mediated disease can then be optimized during the period of B cell depletion. Rituximab treatment for idiopathic membranous nephropathy has been reported. Treatment protocols have involved two regimens: four weekly doses of 375┬Āmg/m2, which is the standard dose for treatment of lymphoma, and two doses of 1┬Āg given at 2-week intervals, which is standard for rheumatoid arthritis. Ruggenenti et al. [2] prospectively treated eight nephrotic patients with persistent proteinuria of >3.5┬Āg/day. Rituximab (375┬Āmg/m2) was administered as four weekly infusions. At 3 months and 12 months, proteinuria significantly decreased from 8.6┬▒4.2 [mean┬▒standard deviation (SD)] to 4.3┬▒3.3┬Āg/day (ŌłÆ51%, P<0.005) and 3.0┬▒2.5 (ŌłÆ66%, P<0.005) g/day, respectively. Fervenza et al. [4] conducted a prospective, open-labeled pilot trial of rituximab treatment in 15 severely nephrotic patients with proteinuria refractory to angiotensin-converting enzyme inhibition and/or receptor blockade. Rituximab (1┬Āg) was administered twice, 2 weeks apart; at 6 months, patients with persistent proteinuria but recovered B cell counts were given a second round of rituximab treatment. Proteinuria was significantly decreased from baseline proteinuria of 13.0┬▒5.7┬Āg/day (range, 6.1ŌĆō23.5┬Āg/day) to 6.0┬▒7.3┬Āg/day at 12 months (ŌłÆ54%; range, 0.2ŌĆō20┬Āg/day; P<0.001).
Fervenza et al. [5] also evaluated the optimal rituximab dose for idiopathic membranous nephropathy, using the same four weekly dose schedule as previously used by Ruggenenti et al. [2] as well as the two doses of rituximab as in their previous work (1┬Āg each; 48% reduction in mean protein levels). At a 12-month follow-up, they found no significant difference in the response rate. For this reason, we treated patients with 1┬Āg of rituximab at 2-week intervals, considering patient convenience and possible difficulties associated with weekly hospital visits for rituximab injections.
In our two cases, rituximab regimens were not only complied with, but were also effective for gradually reducing proteinuria. Previously used immunosuppressant agents, including cyclophosphamide, cyclosporine, mycophenolate mofetil, and steroids, failed to achieve the same outcome. However, the estimated GFR of the first case declined with proteinuria reduction, and proteinuria did not decrease to less than 1┬Āg/day. The results in the first case were not as impressive as in the second case. Given the possible side effects of rituximab, our patients were treated with 100┬Āmg methylprednisolone, 650┬Āmg acetaminophen, and 4┬Āmg chlorpheniramine before rituximab infusion. Although we did not observe any side effects such as flushing or pruritus, other studies have reported mostly mild rituximab infusion reactions in 20ŌĆō40% of cases [11]. The formation of antichimeric antibodies to rituximab is found in approximately 33% of patients with poor B cell depletion. Furthermore, B cell depletion can predispose patients to severe viral infections such as progressive multifocal leukoencephalopathy after rituximab therapy. In 2009, the US Food and Drug Administration reported a third case of progressive multifocal leukoencephalopathy in a patient with rheumatoid arthritis [12].
As illustrated in our two cases, rituximab therapy could be a feasible treatment option with minimal side effects for idiopathic membranous nephropathy patients who are refractory to immunosuppressive therapies. Compared with recent studies, our follow-up periods were relatively short, and a longer follow-up period would be desirable. Ruggenenti et al. reported that remission was achieved over a median of 7.1 months [13]. Another report showed that proteinuria tends to decline slowly and remissions may occur up to 2 years after rituximab treatment [14]. Combinations of rituximab with abbreviated courses of other agents with quicker onset of antiproteinuric effects is under investigation [13].The timing, duration, and specific dosing of rituximab remain unclear. Large, multi-center, randomized trials with long-term follow-up are needed to verify the long-term tolerability and overall survival benefit of rituximab therapy.
References
1. Cattran D. Management of membranous nephropathy: when and what for treatment. J Am Soc Nephrol 16:2005;1188ŌĆō1194.


2. Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, Remuzzi G. Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol 14:2003;1851ŌĆō1857.


3. Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ. Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296:2009;F213ŌĆōF229.


4. Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, Leung N, Cohen IM, Wochos DN, Bergstralh E, Hladunewich M, Cattran DC. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int 73:2008;117ŌĆō125.


5. Fervenza FC, Abraham RS, Erickson SB, Irazabal MV, Eirin A, Specks U, Nachman PH, Bergstralh EJ, Leung N, Cosio FG, Hogan MC, Dillon JJ, Hickson LJ, Li X, Cattran DC. Rituximab therapy in idiopathic membranous nephropathy: a 2-year study. Clin J Am Soc Nephrol 5:2010;2188ŌĆō2198.



6. Quaglia M, Stratta P. Idiopathic membranous nephropathy: management strategies. Drugs 69:2009;1303ŌĆō1317.


7. Ronco P, Debiec H. Antigen identification in membranous nephropathy moves toward targeted monitoring and new therapy. J Am Soc Nephrol 21:2010;564ŌĆō569.


8. Kerjaschki D, Farquhar MG. The pathogenic antigen of Heymann nephritis is a membrane glycoprotein of the renal proximal tubule brush border. Proc Natl Acad Sci USA 79:1982;5557ŌĆō5561.



9. Herrmann SM, Sethi S, Fervenza FC. Membranous nephropathy: the start of a paradigm shift. Curr Opin Nephrol Hypertens 21:2012;203ŌĆō210.


10. Johnson PW, Glennie MJ. Rituximab: mechanisms and applications. Br J Cancer 85:2001;1619ŌĆō1623.



12. http://www.fda.gov/downloads/safety/medwatch/safetyinformation/safety/alertforhumanmedicalproducs/ucm187792.pdf.
Figure┬Ā1
Data from Case 1. (A) Proteinuria was increased to 12┬Āg/day. Rituximab was first administered once daily, 2 weeks apart. Urine protein/creatinine ratio decreased to 1.12┬Āmg/mg after 6 months, but the urine protein/creatinine ratio increased to 5.17┬Āmg/mg soon after. A second round of rituximab was administered after 10 months of follow-up. The urine protein/creatinine ratio decreased to 2.95┬Āmg/mg. Partial remission was achieved. (B) The estimated GFR of Case 1 showed worsening after rituximab infusion. GFR, glomerular filtration rate.
Figure┬Ā2
Data from Case 2. (A) Urine protein/creatinine ratio increased to 7.95┬Āmg/mg. Rituximab was administered once daily, 2 weeks apart. Six months later, rituximab was administered again due to an increase of proteinuria. Partial remission was achieved after rituximab infusion 4 months later. (B) The estimated GFR of Case 2 shows an improvement of 17% after rituximab therapy. GFR, glomerular filtration rate.
- TOOLS
-
METRICS

- Related articles
-
Effect of immunosuppressive agents on clinical outcomes in idiopathic membranous nephropathy
A Case of Crescentic IgA Nephropathy Associated with Alcoholic Liver Cirrhosis2011 November;30(6)
A Case of IgA Nephropathy associated with Acute Hepatitis A2010 January;29(1)
A Case of AA Amyloidosis Treated with Infliximab2010 September;29(5)



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















