A case of reninoma with variant angina
Article information
Abstract
Reninoma is a tumor of the renal juxtaglomerular cell apparatus that causes hypertension and hypokalemia because of hypersecretion of renin. We present a case of a 29-year-old female patient with reninoma and concomitant variant angina. The patient had uncontrolled hypertension and elevated plasma renin activity and aldosterone levels. Imaging studies revealed a mass in the left kidney, which was further confirmed as a renin-producing lesion via selective venous catheterization. During the evaluation, the patient had acute-onset chest pain that was diagnosed as variant angina after a provocation test. After partial nephrectomy, the plasma renin activity and plasma aldosterone levels decreased and blood pressure normalized. We report a case of reninoma with variant angina.
Introduction
Juxtaglomerular cell tumor (JCT) of the kidney, also called reninoma, is a rare benign renal neoplasm that was first described by Robertson et al [1] in 1967. To date, approximately 110 patients with JCT of the kidney have been reported in the English-language literature [2], [3], [4], [5], [6], [7]. These patients typically present with high blood pressure, usually associated with hypokalemia and hyperaldosteronism secondary to renin hyperproduction by the tumor. We report a case of a young woman with reninoma who presented with uncontrolled hypertension and variant angina.
Case report
A 29-year-old woman presented to our nephrology clinic with recurrent headache and dizziness. Five years previously, she was diagnosed with hypertension and evaluated for secondary hypertension, which revealed no abnormal findings. Three years later, elevated plasma renin activity (PRA) and aldosterone levels were detected. Therefore, renovascular hypertension was suspected. The results of a captopril renal scan and computed tomography (CT) angiography were normal, but a 0.8-cm mass was revealed by renal magnetic resonance imaging. However, the association between hypertension and the renal mass was not suspected at that time. One month previously, because of persistent uncontrolled hypertension and recurrent dizziness despite intensive antihypertensive medication, she was re-evaluated with CT at another hospital. Abdominal CT revealed that a renal mass in the left kidney had increased to 1.5 cm (Fig. 1). She was transferred to our nephrology clinic for further evaluation.
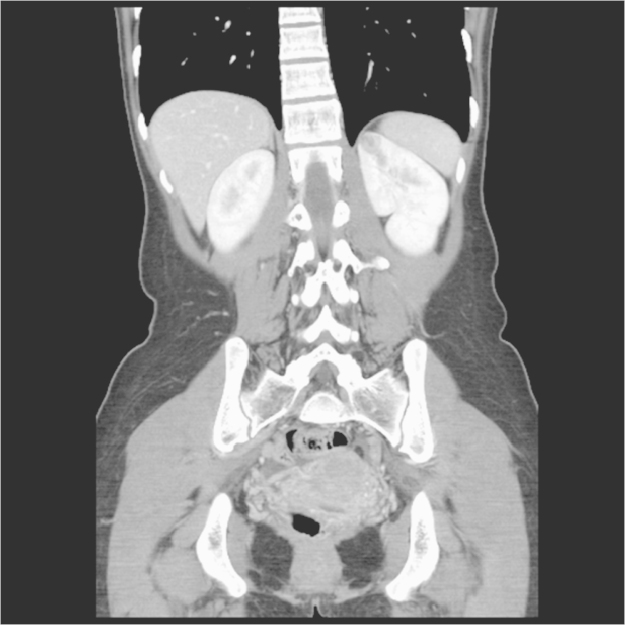
Abdominal computed tomography shows a well-defined 1.5-cm mass in the upper pole of the left kidney.
Her blood pressure at presentation was 170/128 mmHg. Her medications were 8 mg candesartan, 30 mg nifedipine, and 25 mg spironolactone once daily. She underwent appendectomy and reported that her grandfather had hypertension. The patient was 156.8 cm in height and 49.3 kg in weight. Physical examination revealed intact eye fundi and, no evidence of cardiac abnormalities, abdominal bruits, and abdominal masses. Initial electrocardiogram showed normal sinus rhythm. Echocardiographic findings were normal, without evidence of aortic coarctation.
The results of a complete blood count were unremarkable (white blood cell count, 7,030/μL; hemoglobin, 13.2 g/dL; hematocrit, 40.1%; platelet count, 244,000/μL). Hypokalemia was noted (potassium, 2.8 mmoL/L; normal range, 3.5–5.5 mmoL/L). In the supine position, PRA was 31.4 ng/mL/hour (normal range, 1.0–2.5 ng/mL/hour) and aldosterone level was 204.5 ng/dL (normal range, 3.0–16.0 ng/dL). Pheochromocytoma and renovascular hypertension could be excluded by other laboratory findings and image studies. Renal vein sampling test showed that high plasma renin level was originated from renin hypersecretion of the renal mass (Table 1).
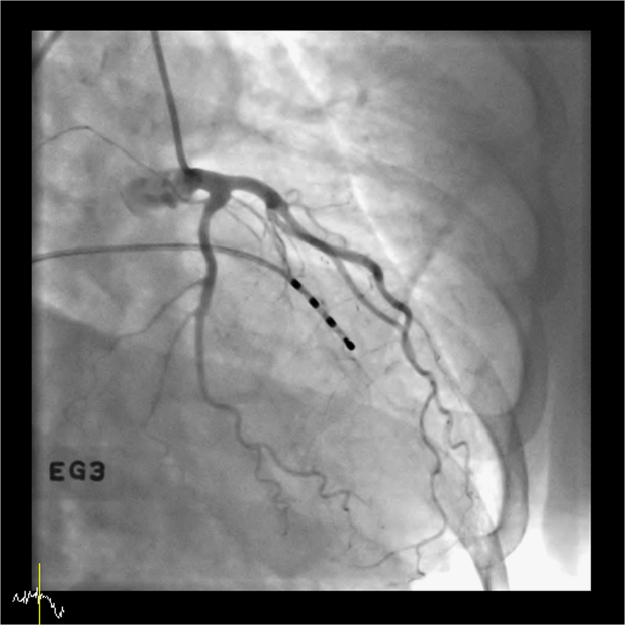
One day after renal vein sampling, the patient experienced severe chest pain of acute onset, which she had not experienced before. An electrocardiogram showed ST depression and the level of cardiac enzyme troponin I was mildly elevated (0.05 ng/mL; normal range, 0–0.028 ng/mL). Coronary angiography did not show significant coronary lesion to chest pain. However, ergonovine provocation test showed diffuse vasospasm of the left circumflex artery (Fig. 2). The patient was diagnosed with variant angina and did not develop chest pain afterwards. The medication given prior to surgery was 8 mg/day candesartan.
A few days later, a well-demarcated, 1.5-cm endophytic mass located on the surface of the upper pole of the left kidney was removed. The surgical procedure was completed success- fully without complications. Pathology results were consistent with JCT, showing clusters of large cells with pale cytoplasm and uniformly round nuclei (Fig. 3). Electron microscopic examination demonstrated characteristic rhomboidal renin granules.
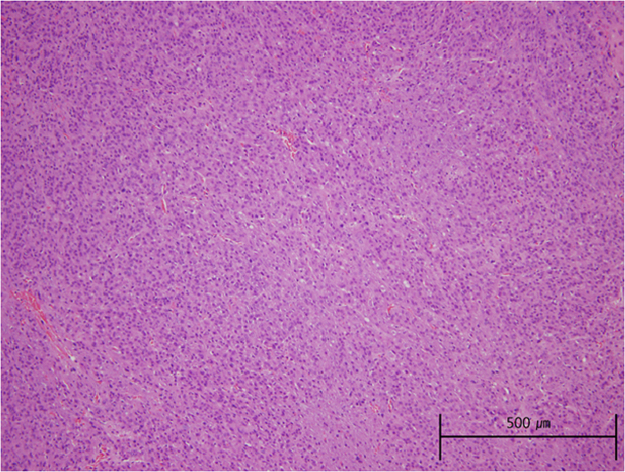
Pathology of the renal specimen. Hematoxylin and eosin stain: densely arranged polygonal tumor cells with abundant eosinophilic cytoplasm and uniform nuclei (100×).
Blood pressure was normalized and she did not chest pain despite no anti-hypertensive and angina medication after nephrectomy (Table 2).
Discussion
JCT of the kidney is a rare and benign renal neoplasm. An epidemiological study revealed that these tumors generally occur in relatively young patients with a mean age of 27 years, predominantly women (male to female ratio, 1:2) [3], [7]. The clinical presentations of JCT include marked hypertension, hyperaldosteronism, and hypokalemia secondary to renin secretion by the tumor. In addition, the patients frequently complain of headache, dizziness, and polyuria [6].
On plain CT, JCT usually appears as a unilateral, well-circumscribed hypoattenuating cortical mass, and this modality is the most useful tool for the detection of a renal mass, with a sensitivity approaching 100% [6]. On magnetic resonance imaging, JCT may appear as a cortical-based renal mass of variable signal intensity, which provides useful diagnostic information [8].
Any mass detected using the above imaging modalities must be confirmed by renal vein sampling and surgical pathology. Venous sampling for renin assay is helpful for the diagnosis and localization of the tumor. The criteria for defining PRA lateralization during renal vein sampling are unclear. In a previous study, a lateralization ratio of 1.5 was shown to maximize specificity while limiting sensitivity [6]. Blood samples are obtained by interventional angiography. Regarding surgical management, nephron-sparing surgery with tumor resection should be considered.
Although myocardial infarction associated with reninoma and myocardial bridging has been reported previously [9], there are no case of variant angina associated with reninoma. In the present case, the patient experienced chest pain of acute onset and was diagnosed with variant angina. The mechanism of variant angina is coronary vasospasm, although the cause of vasospasm remains unknown. It is speculated to be related to dysfunction of coronary artery endothelium. After removal of the renin-secreting tumor, no episodes of chest pain were reported. Alderman et al [10] have reported that the renin profile is independently associated with the subsequent risk of myocardial infarction. The present case is anecdotal, thus, the association between hyper-reninemia and variant angina needs to be further investigated from other case reports or case series.
Reninoma is a rare disease contributing to the development of secondary hypertension. Although uncommon, a renin-producing tumor is a treatable cause of hypertension, and thus should be suspected in any patient with an elevated aldosterone level associated with refractory hypertension, especially in the presence of hypokalemia [11].
Conflicts of interest
All the authors declare no competing interests.