The influence of hypophosphatemia on outcomes of low- and high-intensity continuous renal replacement therapy in critically ill patients with acute kidney injury
Article information
Abstract
Background
The purpose of this study was to assess the role of hypophosphatemia in major clinical outcomes of patients treated with low- or high-intensity continuous renal replacement therapy (CRRT).
Methods
We performed a retrospective analysis of data collected from 492 patients. We divided patients into two CRRT groups based on treatment intensity (greater than or equal to or less than 40 mL/kg/hour of effluent generation) and measured serum phosphate level daily during CRRT.
Results
We obtained a total of 1,440 phosphate measurements on days 0, 1, and 2 and identified 39 patients (7.9%), 74 patients (15.0%), and 114 patients (23.1%) with hypophosphatemia on each of these respective days. In patients treated with low-intensity CRRT, there were 23 episodes of hypophosphatemia/1,000 patient days, compared with 83 episodes/1,000 patient days in patients who received high-intensity CRRT (P < 0.01). Multiple Cox proportional hazards analysis showed that Acute Physiology and Chronic Health Evaluation (APACHE) III score, utilization of vasoactive drugs, and arterial pH on the second day of CRRT were significant predictors of mortality, while serum phosphate level was not a significant contributor to mortality.
Conclusion
APACHE score, use of vasoactive drugs, and arterial pH on the second CRRT day were identified as significant predictors of mortality. Hypophosphatemia might not be a major risk factor of increased mortality in patients treated with CRRT.
Introduction
Phosphate level is an important aspect of normal physiology, with phosphate serving as an essential component of structural cell membrane molecules (e.g., phospholipids) and second messengers (e.g., adenosine mono-phosphate) and as a vital energy source (e.g., adenosine triphosphate) [1]. The normal mechanisms that regulate phosphate level are disrupted by acute critical illnesses, including acute kidney injury (AKI) [2], and hypophosphatemia is one of the most frequently encountered electrolyte disorders in critically ill patients [3,4]. Impaired energy metabolism is the common mechanism for hypophosphatemia-induced complications; as a result, hypophosphatemia has been described as a metabolic disturbance leading to cellular dysfunction in multiple organ systems [1].
Treatment of AKI with continuous renal replacement therapy (CRRT) can result in hypophosphatemia secondary to excess phosphate removal [5]. However, there is limited information regarding the timing, severity, duration, and independent associations of hypophosphatemia with clinical outcomes. While many studies have shown an association between hypophosphatemia and increased mortality [6,7], it remains unclear whether hypophosphatemia contributes to mortality or is merely a marker of illness severity. Clarification of the relationship between hypophosphatemia and mortality is especially important for high-intensity CRRT due to the reportedly greater incidence of hypophosphatemia seen in recent dialysis trials [8,9]. Additionally, two recent analyses of cohort data showed that hypophosphatemia during RRT is associated with prolonged respiratory failure [10] and increased risk of in-hospital mortality [11].
If hypophosphatemia independently contributes to a greater risk of mortality or morbidity, it might account for its more frequent occurrence in high-intensity CRRT. Thus, the purpose of this study was to assess whether hypophosphatemia is independently associated with major clinical outcome in patients treated with low- or high-intensity CRRT.
Methods
Patients
We retrospectively reviewed the cases of approximately 4,500 critically ill patients brought to the intensive care unit (ICU) of one of three tertiary hospitals between August 2012 and October 2014. Among the cases reviewed, we identified 492 individuals at least 18 years of age who were diagnosed with AKI and who were receiving CRRT: these patients served as the patient group for the present study. Patients were divided into two major groups based on CRRT intensity, comprising a low-intensity (< 40 mL/kg/hour) CRRT group with 414 patients and a high-intensity (≥ 40 mL/kg/hour) CRRT group with 78 patients. Serum phosphorus level was measured at least once daily at 6:00 a.m. Patients with hypophosphatemia were administered a sodium glycerophosphate injection according the drug manufacturer’s instructions. All procedures performed in studies involving human participants were in accordance with the ethical standards of the respective tertiary hospital, the 1964 Helsinki declaration and its later amendments, or comparable ethical standards. Institutional review board approval was obtained prior to the start of the study (No. 15-039).
Inclusion and exclusion criteria
Patients were included in our study if they were critically ill, at least 18 years of age, diagnosed with AKI, deemed by the treating clinicians to require CRRT, and had at least one of the following signs [12] based on the RIFLE (risk, injury, failure, loss, end stage) criteria: a > 2-fold increase in serum creatinine or urine output < 0.5 mL/kg/hour for 12 hours.
In addition, patients needed to have at least one of the following criteria:
1) Clinically significant organ edema (e.g., pulmonary edema)
2) Oliguria (urine output < 200 mL/12 hours)
3) Azotemia (blood urea nitrogen [BUN] > 70 mg/dL)
4) Hyperkalemia (K > 6.5 mmol/L)
5) Severe metabolic acidosis (pH < 7.1)
The following subjects were excluded from our analysis:
1) Patients who had received 24 hours or more of CRRT by the time of enrollment
2) Patients who weighed < 50 kg or >120 kg because of the limitations of the Prisma®machine (Gambro, Medolla, Italy) to deliver appropriate study doses for those weights
3) Patients with non-renal indications for CRRT
4) Patients whose hospital stay was < 24 hours or who had been readmitted
5) Patients with underlying phosphorus disturbance stemming from a premorbid condition
6) Patients with a current or past history of cancer
7) Patients with end-stage renal disease requiring dialysis
The criteria for exclusion were hypoparathyroidism, hyperparathyroidism, chronic renal tubular defects, alcoholic ketoacidosis, diabetic ketoacidosis, or persistent respiratory alkalosis
Recovery of renal function was defined as a lack of need for renal replacement therapy and estimated creatinine clearance greater than 60 mL/min/1.73 m2 at the time of hospital discharge [13].
Clinical and biochemical data collection
Demographic and clinical data were recorded at the time of CRRT initiation. Biochemical data for electrolyte and mineral levels including sodium, potassium, calcium, and phosphate were measured before starting CRRT (0 hour) and again 24 and 48 hours after initiating CRRT.
Treatment modality
All CRRT sessions were conducted using Prisma dialysis machine (Gambro) with M 100 (acrylonitrile + sodium methallyl sulfonate) or ST 100 (acrylonitrile + sodium methallyl sulfonate + polyethylene imine) membrane dialyzers. A 12-F double-lumen catheter was inserted into the internal jugular, subclavian, or femoral vein, and the pre- or post-dilution method was used for replacement fluid infusion. CRRT was initiated and managed by the attending nephrologist. Bicarbonate-based replacement solutions were utilized. Heparin was used unless contraindicated, in which case, nafamostat mesilate was used instead. Body weights were estimated using the usual body weight. Venous pressure, X-ray findings, and the difference between total input and output because body weights were not measurable due to patient condition. Replacement fluid in the post-dilution modality was typically infused at a rate of 1–2 L/hour, and the CRRT dose equated to an average of 35 mL/kg/hour (range, 30–42 mL/kg/hour) comprising the replacement fluid rate + dialysate flow rate using continuous veno-venous hemodiafiltration (CVVHDF). The blood flow rate was 80–180 mL/minute, and the dialysate flow rate was 1–2 L/hour. The dialysis dose was calculated as the total effluent discharged per day divided by the patient’s baseline weight for the first 3 days, thus taking into account downtime due to interruptions during 24-hour dose delivery [14].
Variables
The study endpoints consisted of death during the 90-day period and survival 90 days after initiation of CV-VHDF. Hypophosphatemia was defined as a serum phosphate level less than 2.5 mg/dL.
Statistical analysis
Data are expressed as the mean ± standard deviation. Discrete categorical variables were compared using the chi-square test, and continuous variables were compared with the Student’s t-test. Predictors of all-cause mortality were examined using Kaplan–Meier and Cox proportional hazards analysis in both treatment groups. Cox proportional hazards analysis was used to determine the independent contribution of prognostic factors for predicting outcomes 90 days after initiating CVVHDF therapy. For multivariate analyses, a significance level of 0.10 was used for exclusion and inclusion in the model and yielded no differences in the model output. Candidate predictors, namely, those factors that were statistically significant in the univariate model, were sex, Acute Physiology and Chronic Health Evaluation (APACHE) III score, chronic kidney disease, oliguria, mechanical ventilation, sepsis, diabetes mellitus, cardiovascular disease, degree of organ failure, use of vasoactive medications, CCRT effluent flow rate, arterial pH, and serum phosphate. Multivariate Cox analysis was performed after excluding the APACHE III components, age, and first arterial pH.
Results were considered significant when the P value was < 0.05. Statistical analyses were performed using the PASW Statistics software version 18.0 (IBM Co., Armonk, NY, USA).
Results
Table 1 shows the baseline characteristics of patients according to CRRT dose. The mean age was 63.1 (± 13.7) years in the low-dose group and 62.6 (± 12.1) years in the high-dose group. Medical diseases (71.7% in the low-dose group, 71.6% in the high-dose group) were the most common clinical indications for CRRT. Diabetes mellitus was the most common underlying disease in the high- and low-dose CRRT groups (42.2% in the low-dose group, 46.2% in the high-dose group). The proportion of patients with CKD was greater in the high-dose group (43.0%) than the low-dose group (28.7%). Multiple organ failure was the most common cause of death (59.5% in the low-dose group, 50.0% in the high-dose group), followed by cardiac causes. Hospital mortality at 90 days was higher in the low-intensity CRRT group (53.3%) compared to the high-intensity CRRT group (43.7%), although the difference did not reach statistical significance.
Table 2 summarizes the baseline characteristics and outcomes of patients. Similar to the results described above, there were more patients with CKD in the higher serum phosphorus group (35.6%) than the lower phosphorus group (21.3%), although there were no significant differences between the two groups. Patients with hypophosphatemia at the time of starting CRRT had similar outcomes. We identified 39 patients (7.9%), 74 patients (15.0%), and 114 patients (23.1%) with hypophosphatemia on days 0, 1, and 2, respectively. A total of 139 patients developed hypophosphatemia during CRRT. The number of patients with hyperphosphatemia on days 0, 1, and 2 were 186 (37.8%), 155 (31.5%) and 136 (27.6%), respectively. There was no significant difference in baseline characteristics between the three groups (i.e., serum phosphorus < 2.5, 2.5 ≤ serum phosphorus < 4.5, and serum phosphorus ≥ 4.5). In addition, presence of hyperphosphatemia (serum phosphorus ≥ 4.5) was not associated with mortality.
Ischemia or shock (40.7% in the low-dose group, 52.3% in the high-dose group) was the most common etiology of AKI, followed by sepsis (Table 3). Other causes included nephrotoxins, rhabdomyolysis, and urinary tract obstruction. Among the reasons for initiating CRRT, oliguria or anuria (45.8% in the low-dose group, 42.0% in the high-dose group) was the most common, followed by fluid overload, as noted in Table 4. Fluid overload was estimated by central venous pressure, X-ray findings, and the difference between total input and output, because body weights were not measurable due to patient condition. High BUN/Cr ratio, metabolic acidosis, and hyperkalemia were additional causes of AKI.
Table 5 shows the characteristics pertaining to CRRT itself. The number of days prior to starting CRRT was smaller in the high-dose group (6.5 ± 10.2) compared to the low-dose group (8.1 ± 12.5). CVVHDF was the mode of CRRT in all patients, and the mean effluent flow rate was 31.4 ± 3.0 mL/kg/hour and 50.2 ± 8.8 mL/kg/hour in the high- and low-CRRT groups, respectively.
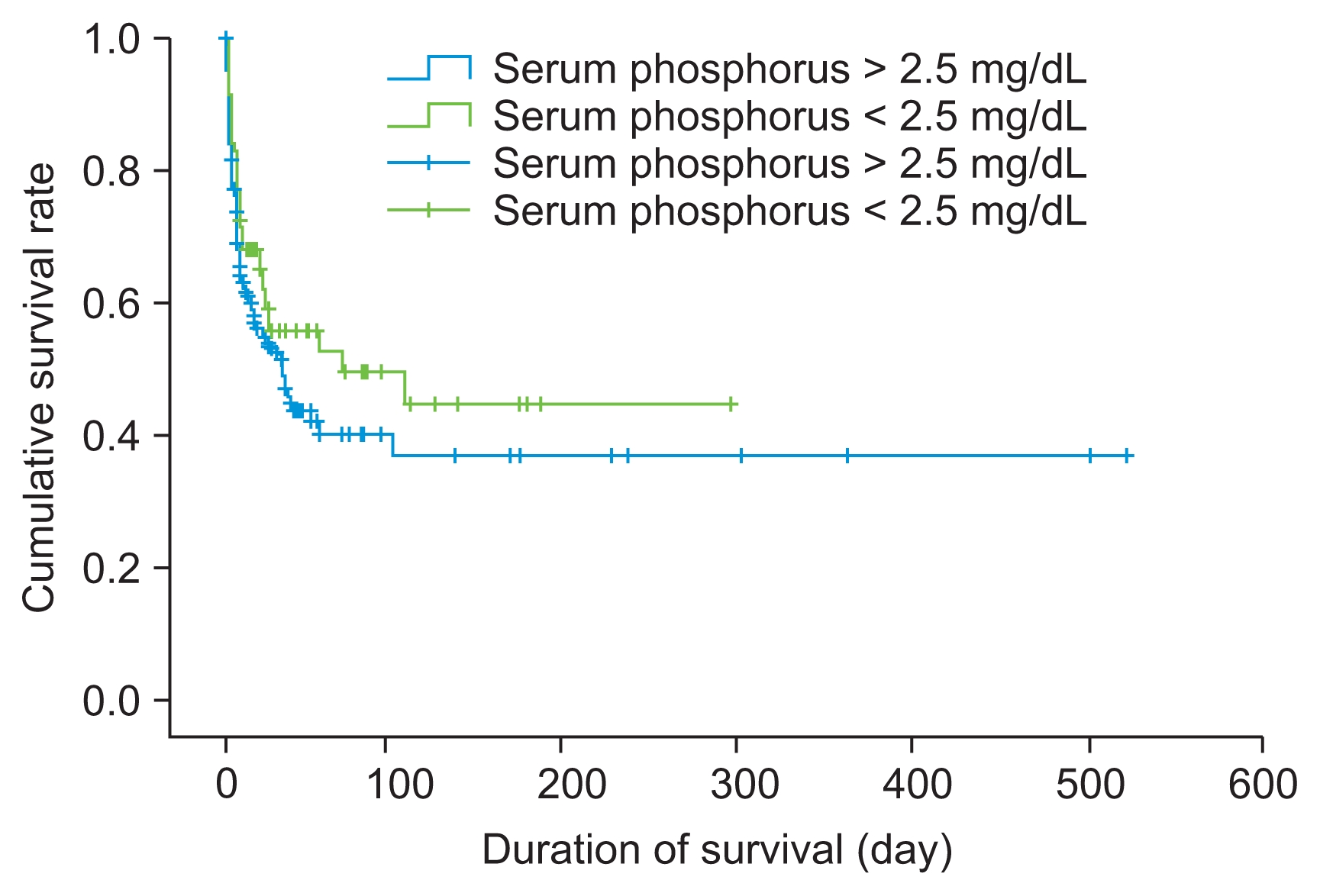
Table 6 shows that there was no significant difference between groups with respect to clinical status at the conclusion of CRRT (e.g., recovery of renal function and death). The multiple Cox proportional hazards analysis at the zeroth CRRT day showed that APACHE III score and utilization of vasoactive drugs were significant predictors of mortality (Table 7), and multiple Cox proportional hazards analysis at the second CRRT day showed that APACHE III score, utilization of vasoactive drugs, and arterial pH on the second CRRT day were significant predictors of mortality (Table 8), while serum phosphate level did not contribute significantly to mortality. Finally, as shown in Fig. 1, the 28-day and 90-day survival rates were 60.3% and 55.3% in the high-dose group and 58.3% and 41.6% in the low-dose group, respectively (P = 0.050). In addition, Fig. 2 shows that the 90-day survival rate was 52.2% in the hypophosphatemia group and 46.6% in the non-hypophosphatemia group (P = 0.080).

Multivariate Cox regression analysis of factors associated with mortality at zero continuous renal replacement therapy (CRRT) day

Multivariate Cox regression analysis of factors associated with mortality at 2nd continuous renal replacement therapy (CRRT) day

Patient survival rate in the low- and high-intensity continuous renal replacement therapy (CRRT) groups (P = 0.048).
Discussion
In the present study, we found that patients with hypophosphatemia had a lower adjusted mortality rate than those without an episode of hypophosphatemia. Therefore, hypophosphatemia might not be a major risk factor of increased mortality in patients treated with CRRT.
There are three main mechanisms that lead to hypophosphatemia: decreased intestinal absorption, increased renal excretion, and internal redistribution of inorganic phosphate. The main causes of hypophosphatemia in critically ill patients include severe infection, trauma, postoperative state, malnutrition, respiratory alkalosis, and diabetic ketoacidosis [1]. In addition, inorganic phosphate redistribution across the cell membrane is a common phenomenon among critically ill patients [15]. Moreover, critically ill patients commonly present with several conditions, including sepsis, alcohol withdrawal, malnutrition, rhabdomyolysis, and elevated catecholamines, all of which require treatments that can predispose to hypophosphatemia, such as intravenous glucose infusion, hyperventilation, and diuretics [16]. Another factor that contributes to hypophosphatemia in CRRT is the technique itself, as it produces a high clearance of small solutes including phosphate [17]. Low serum phosphate level can also occur in the setting of intracellular shifts due to respiratory alkalosis, high blood concentrations of stress hormones (i.e., insulin, glucagon, adrenalin, cortisol), and refeeding syndrome. Therefore, serum phosphorus level does not accurately reflect total body phosphorus stores, and this bias can be aggravated by CVVHDF therapy due to the rapid serum phosphorus clearance that occurs.
In the present study, we showed that a single test of serum phosphorus concentration could not serve as an independent factor predictive of mortality. Recently, the ratio of hypophosphatemic CRRT days to total number of CRRT therapy days has come into favor as a measurement that avoids the bias of serum phosphorus tests. Furthermore, a recent multivariate analysis showed that this ratio is an independent predictor of global clinical outcomes [18].
Severe infection, trauma, postoperative state, and malnutrition have been cited as the main causes of hypophosphatemia in critically ill patients [1]. Indeed, in the present study, 177 patients (28.5%) had received surgical interventions, and 316 patients (50.9%) were diagnosed with sepsis. Therefore, hypophosphatemia might have been present in some patients before initiation of CRRT therapy due to their respective illnesses. Importantly, our study results do not exclude the potential use of hypophosphatemia as a surrogate marker of other conditions, even during the CRRT therapy period. However, the exact frequency and severity of hypophosphatemia in critically ill patients with AKI prior to the initiation of RRT are difficult to determine. First, non-daily measurements of morning serum phosphate might underestimate the prevalence of hypophosphatemia. Secondly, the frequency and severity of acute phosphate disorders are dependent on the characteristics of the ICU population (e.g., nature of the illness and prevalence of preexisting chronic kidney disease) [19]. In addition, renal replacement therapy can also lead to hypophosphatemia, particularly if administered for a prolonged period [20,21].
The higher incidence of hypophosphatemia in the intensive CRRT group in our study was consistent with the findings of two recent, large-scale clinical trials. Specifically, the incidence of hypophosphatemia was reported to range from 54.0% to 65.1% in the Randomized Evaluation of Normal vs. Augmented Level (RENAL) Replacement Therapy Trial and from 10.9% to 17.6% in the VA/NIH Acute Renal Failure Trial Network (ATN) Study [8,9]. Interpreting the results of these two studies strongly implies that phosphate clearance during CVVHDF is significantly higher than during intermittent hemodialysis, possibly due to ongoing intercompartmental mass transfers and larger filter pore size. CVVHDF was used as the modality for renal replacement therapy in the present study, and the incidence of CVVHDF-associated hypophosphatemia matched that of the ATN Trial.
Renal replacement therapy is one of the most widely used methods to treat critical illnesses, especially AKI. Thus, it is crucial to study the effects of the high-incidence complications of AKI on survival, which includes hypophosphatemia. In the present study, 30.3% of the patients presented with hypophosphatemia during CRRT. In addition, the level of serum phosphate in the high-intensity CRRT group was similar to that in the low-intensity group. A possible explanation for this lack of difference between the two groups was the fact that sodium glycerophosphate was administered to all patients with hypophosphatemia. However, changes in level of serum phosphorus after sodium glycerophosphate injection were not tracked as part of this study, and we did not obtain data regarding the treatment of hypophosphatemia in the patients enrolled in this study. There are currently no widely agreed upon guidelines regarding the approach to hypophosphatemia in critically ill patients with AKI who require RRT. However, other studies have shown that administering intravenous phosphate or phosphate-containing dialysis solution in response to hypophosphatemia [21,22] reduces the variability of serum phosphate level during CRRT and eliminates the incidence of hypophosphatemia.
The results of the present study should not be taken to imply that hypophosphatemia is of little consequence or should be left uncorrected. Although our results did not demonstrate a statistically significant association between hypophosphatemia and increased mortality, hypophosphatemia is still an undesirable outcome, and treatment remains indicated in the clinical environment in which we collected our data. On the other hand, our findings might suggest that, in a clinical setting where the occurrence of hypophosphatemia is detected by at least daily measurement and is corrected by phosphate supplementation, there is no independent relationship between hypophosphatemia and increased risk of death.
The association between acute hypophosphatemia and outcome is poorly understood. Acute hypophosphatemia due to phosphate redistribution alone might be of little consequence in the absence of phosphate depletion [1]. Likewise, the cause-and-effect relationship of hypophosphatemia with morbidity and mortality has been difficult to establish [23]. Other studies regarding the association between acute hypophosphatemia and outcome have been small, utilized different definitions of hypophosphatemia, and did not assess the independent relationships between hypophosphatemia and outcomes after adjusting for illness severity. A recent cohort study of ICU patients treated with CRRT looked for an independent association between hypophosphatemia and mortality [10] and, consistent with the results of our study, found no association between hypophosphatemia and mortality after adjusting for illness severity. Finally, a recent large study of 2,730 critically ill patients also found no independent relationship between hypophosphatemia and patient outcome [24].
Identifying clinical consequences of acute severe hypophosphatemia in critically ill patients with multi-organ dysfunction is difficult. Specifically, the respiratory, cardiovascular, neurological, skeletomuscular, and hematologic effects of hypophosphatemia can often be attributed to the nature and severity of the acute illness and/or exacerbation of preexisting disease. However, recent reports of cohort study data suggest that hypophosphatemia during RRT should no longer be considered a harmless therapy-induced-side effect, as it can be associated with prolonged respiratory failure [10] and increased risk of in-hospital mortality [11].
Several studies have reported an association between hypophosphatemia and increased mortality [6,25]. Unfortunately, there is little epidemiological data regarding the efficacy of replacement therapy-associated hypophosphatemia and global clinical outcomes. A few recent studies have shown that high-dose CVVHDF does not improve patient outcomes [26,27]. In our study, although mortality at 90 days was high in the low-intensity CRRT group (53.3%) compared to the high-intensity CRRT group (43.7%), the difference did not reach statistical significance.
There were several limitations of the present study. First, data were retrieved retrospectively from the databases of three tertiary care hospitals. Thus, the association between outcome and female sex might have been a consequence of decreased enrollment of female patients in the study. For example, if dialysis dose was rounded in some cases, which is a common practice, female patients might have tended to receive a higher dose of CRRT compared to men. This might have exaggerated the association between female sex and hypophosphate-mia, suggesting that females are more prone to develop hypophosphatemia during CRRT in ICU, when instead it might have been a consequence of more phosphorus being removed from females due to a higher CRRT dose. A second limitation of this study was that, although phosphate measurements were obtained in patients on CRRT in our institution, subsequent data on hypophosphatemia, though likely low in prevalence, was assumed to be missing at random. In addition, this study did not use a standardized protocol for serum phosphate replacement, which might have confounded our analysis of hypophosphatemia.
A third limitation of this study was that because body weight was not measurable due to patient condition, body weight was estimated based on usual body weight, venous pressure, X-ray findings, and the difference between total input and output. Other methods to estimate actual body weight in an emergency setting include abdominal and thigh circumference [28]. The use of such anthropometric measurements in the future could potentially prove useful to accurately predict a patient’s weight, improve drug dosing, and reduce the number of medication errors. A final limitation of this study was the possibility of various clinical settings leading to hypophosphatemia in critically ill patients. Thus, we are unable to exclude the possibility that hypophosphatemia is a surrogate marker of something other than CRRT based on these findings. A prospective study with a wider range of survival days would be useful in resolving this bias.
In conclusion, APACHE score, use of vasoactive drugs, and arterial pH on the second CRRT day were significant predictors of mortality. Hypophosphatemia might not be a major risk factor of increased mortality in patients treated with CRRT; however, a prospective, multi-center, controlled trial is needed to confirm these findings.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.