Comparison of dominant and nondominant C3 deposition in primary glomerulonephritis
Article information
Abstract
Background
Alternative complement pathway dysregulation plays a key role in glomerulonephritis (GN) and is associated with C3 deposition. Herein, we examined pathological and clinical differences between cases of primary GN with C3-dominant (C3D-GN) and nondominant (C3ND-GN) deposition.
Methods
We extracted primary GN data from the Korean GlomeruloNEphritis sTudy (KoGNET). C3D-GN was defined as C3 staining two grades greater than C1q, C4, and immunoglobulin via immunofluorescence analysis. To overcome a large difference in the number of patients between the C3D-GN and C3ND-GN groups (31 vs. 9,689), permutation testing was used for analysis.
Results
The C3D-GN group exhibited higher serum creatinine (p ≤ 0.001), a greater prevalence of estimated glomerular filtration rate of <60 mL/min/1.72 m2 (p ≤ 0.001), higher (but not significantly so) C-reactive protein level, and lower serum C3 level (p ≤ 0.001). Serum albumin, urine protein/creatinine ratio, number of patients who progressed to end-stage renal disease, and all-cause mortality were comparable between groups. Interstitial fibrosis and mesangial cellularity were greater in the C3D-GN group (p = 0.04 and p = 0.01, respectively) than in the C3ND-GN group. C3 deposition was dominant in the former group (p < 0.001), in parallel with increased subendothelial deposition (p ≤ 0.001).
Conclusion
Greater progression of renal injury and higher mortality occurred in patients with C3D-GN than with C3ND-GN, along with pathologic differences in interstitial and mesangial changes.
Introduction
The complement system is a major host defense mechanism of innate and adaptive immunity, with complement proteins synthesized and secreted in response to various stimuli [1]. The kidney is susceptible to complement-associated injury, which is most frequently triggered by immune complex (IC) deposition and classic complement pathway activation but can also be induced via activation of the alternative pathway (AP) in the absence of IC deposition [2]. Deficiency of specific components or defective complement regulation at different sites results in various manifestations of disease, clinical features, and outcomes [3].
Recently, C3 glomerulopathy (C3G) was designated as a unique pathological entity that includes a spectrum of diseases with predominant glomerular C3 fragment deposition in the near absence of C1q, C4, and immunoglobulins (Ig) under immunofluorescence (IF) analysis, with the underlying pathogenesis being driven by overactivation of the AP system [4–6]. The C3 protein plays a central role in C3G pathophysiology through its enzymatic cleavage into C3a and C3b. Of these, C3a is an anaphylatoxin and cytokine precursor, while C3b forms AP C3 convertase and amplifies the C3 activation loop. Through C5 convertase activation by AP C3 convertase, C5a and C5b are generated, with the latter leading to formation of the membrane attack complex. C3G can be subdivided into dense deposit disease (DDD) and C3 glomerulonephritis (C3GN) based on electron microscopy (EM) findings. In DDD, EM reveals linear, hyperosmophilic deposits along the glomerular basement membrane and in the mesangium, while mesangial and/or subendothelial, intramembranous, and subepithelial deposits are present in C3GN. Additionally, DDD is associated with more aggressive clinical outcomes, such as end-stage renal disease (ESRD) [7,8].
Although C3G is confirmed by pathological findings, different features may appear under light microscopy (LM), and variable amounts of Ig can be detected via IF. C3G is considered a disease process rather than just a result of biopsy analysis [4]. Thus, we retrospectively redefined glomerulonephritis (GN) with C3-dominant deposition (C3D-GN) based on IF findings among primary GN cases from a multicenter database in Korea. Our aim was to investigate and compare the clinical characteristics, pathological findings, and long-term outcomes of C3D-GN in primary GN with those of non-C3 dominant GN (C3ND-GN).
Methods
This study was conducted in accordance with the 1964 Declaration of Helsinki and approved by the Institutional Review Board (IRB) of Seoul National University Hospital (No. B-1707/408-106). The need for informed written consent was waived by the IRB because of the retrospective nature of the study and the minimal risk to participants.
Patients and data collection
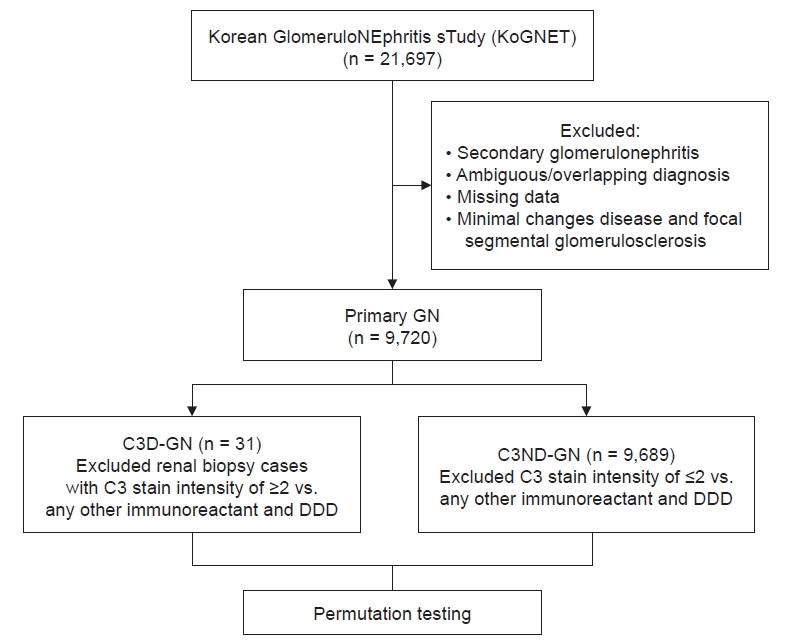
This retrospective study used information from the Korean GlomeruloNEphritis sTudy (KoGNET), which is a database containing information from 21,697 patients who underwent renal biopsies at 18 centers across Korea between 1979 and 2018. In all cases, routine analyses including LM, IF, and EM were performed, and a renal pathologist established the diagnosis at each hospital. IF findings were graded on a scale of 0–4, as follows: 0, trace; 1+; 2+; 3+; and 4+. All clinical data at the time of biopsy and the last follow-up were saved in the hospital information system. We retrieved medical records to obtain data on demographic and clinical features of age, sex, body mass index (BMI), underlying disease, renal function, proteinuria, serum C3, serum C4, and outcomes (ESRD and all-cause mortality).
Proteinuria was evaluated by a 24-hour quantitative measurement. The estimated glomerular filtration rate (eGFR) was calculated based on the original Modification of Diet in Renal Disease equation. Data on ESRD and mortality were extracted from each hospital’s information system and the ESRD registry of the Korean Society of Nephrology and the Statistics Korea, respectively. The median duration of follow-up was 99.03 ± 116.33 months for ESRD and 105.46 ± 116.16 months for mortality.
Definition of C3D-GN
Pathological findings were reviewed based on the evaluation findings of the pathologist at each center. Cases for which complete IF findings were not available were excluded. We extracted cases diagnosed with primary GN based on histologic findings in renal biopsy. Primary GN was categorized as minimal change disease (MCD), IgA nephropathy (IgAN), focal segmental glomerulosclerosis (FSGS), membranous GN (MN), and membranoproliferative GN (MPGN) without an evident cause such as viral disease (hepatitis B and C) or systemic disease. Except for all ambiguous or overlapping cases and missing data, only those diagnosed with primary GN were sorted. Both MCD and FSGS were excluded from the C3D-GN reclassification because the diagnosis is highly likely to change if C3 is the dominant deposit in those diseases. If the authors were unable to review the pathology slides anew, those diagnoses were excluded.
In principle, C3G can be defined based on IF and EM findings [9]. For a more precise definition, genetic testing or a complement system evaluation is needed; however, we could not obtain such data. Therefore, C3D-GN was defined as C3-dominant when C3 staining was at least two grades stronger than any combination of C1q, C4, IgG, IgM, and IgA by IF, similar to the definition of C3G. Owing to the large difference in patient counts between groups (50 C3D-GN patients vs. 13,070 C3ND-GN patients), we performed permutation tests for analyses (Fig. 1).
Definition of histologic findings
Glomerular findings included sclerosis, crescent formation, ischemic injury, and mesangium cellularity. Sclerosis and crescent formation were defined as positive if the findings were greater than 10% of glomeruli. Ischemic injury was defined as positive if it was noted in LM findings. Interstitial fibrosis, inflammation, and tubular changes were graded, while vascular changes were defined as positive if atherosclerosis and intimal thickening were noted in LM findings. EM findings were defined as positive if mesangial, subendothelial, and subepithelial deposition were noted.
Statistical analysis
For demographic, clinical, and laboratory findings, continuous variables are expressed as mean values, while categorical variables are expressed as prevalence rates. There was a large difference in patient count between the groups, and we used permutation testing to evaluate whether the differences between the study groups were significant. We performed a permutation test of the differences in baseline characteristics of C3ND-GN patient groups. The permutation test was performed on 10,000 permutations. In the permutation test, the two groups were assumed to be identical under the null hypothesis. Therefore, a random sample of 100 patients was selected among the permutations and randomly divided into two groups as many as 31 patients (C3ND-GN). For continuous variables, the averages are computed, and their differences are recorded. Then, p-values were calculated as the proportion of permutations with an absolute difference larger than that of our whole data. The permutation test for categorical variables was conducted in a similar way except that the difference in proportions for each category was computed and averaged over the categories. All analyses were conducted using R version 4.0.5 (R Foundation for Statistical Computing).
Results
The mean age of the C3ND-GN group was 41.41 ± 16.27 years, and that of the C3D-GN group was 38.92 ± 20.31 years. The proportion of males was 54.2% in the C3ND-GN and 58.1% in the C3D-GN group. The BMI was comparable between groups at approximately 24 kg/m2. There were more diabetic patients and fewer cancer patients in the C3D-GN group than in the C3ND-GN group (16.1% vs. 9.0% and 0% vs. 8.3%, respectively) (Table 1).
Clinical differences
Systolic blood pressure was significantly higher in the C3D-GN group than in the C3ND-GN group (135.37 ± 23.90 mmHg vs. 125.80 ± 18.16 mmHg, p = 0.005). Hemoglobin and albumin levels were similar between the groups. Complement C3 level was within the normal range in both groups but lower in the C3D-GN group than in the C3ND-GN group (76.73 ± 40.69 mg/dL vs. 108.13 ± 26.86 mg/dL, p < 0.001). There was no difference in C4 level between groups. The level of C-reactive protein, a marker of inflammation, tended to be higher in the C3D-GN group than in the C3ND-GN group (2.02 ± 5.17 mg/dL vs. 1.07 ± 4.25 mg/dL, p = 0.097) (Table 2).
With regard to renal injury, serum creatinine (Cr) level in the C3D-GN group suggested the presence of greater damage than in the C3ND-GN group (2.17 ± 3.52 mg/dL vs. 1.20 ± 1.01 mg/dL, p < 0.001). The eGFR tended to be low and there were significantly more patients with eGFR of <60 mL/min/1.72 m2 in the C3D-GN group than in the C3ND-GN group (43.3% vs. 26.8%, p ≤ 0.001). There was no significant difference between the groups in terms of number of patients with proteinuria of >3.5 g/Cr (Table 2).
For ESRD and all-cause mortality, the number of C3GN patients who progressed to ESRD (19.4%) and the all-cause mortality rate (6.5%) tended to be higher in the C3D-GN group (vs. 11.2% and 1.5% in the C3ND-GN group), although the difference was not significant.
Histological differences
Among all primary GN cases, GN defined as C3D-GN was most frequent among cases of MPGN (61.3%), followed by cases of IgAN (25.8%) and MN (12.9%) (Table 3, 4).
There was no significant difference in glomerular change between groups. Rates of global sclerosis, crescent formation, and ischemic injury tended to be similar in the C3D-GN and C3ND-GN groups. However, there was a significant pattern of greater proliferation of mesangial cellularity in the C3D-GN group (Table 5, 6).

Pathological light microscopy findings in the interstitium and tubule of C3D-GN and C3ND-GN patients
Compared to the C3ND-GN group, the C3D-GN group exhibited a significantly greater rate of interstitial fibrosis (above moderate, 8.0% vs. 10.0%; p = 0.04). Although not significant, vascular atherosclerosis and intimal thickening were more severe in the C3D-GN group (Table 7).
Regarding complement and Ig deposition as evaluated by IF, notable C3 deposition was observed in the C3D-GN group. IgA was more frequent in the C3ND-GN group, but the C3D-GN group also exhibited trace and grade 1 deposition of IgA. IgM and IgG were deposited up to grades 1 and 2 in the C3D-GN group, which was comparable to observations in the C3ND-GN group. Light chains were deposited at various grades in both groups (Table 8).
EM revealed that the C3D-GN group had greater subendothelial deposition than the C3ND-GN group (48.4% vs. 12.0%, p < 0.001). Additionally, subepithelial deposition and podocyte effacement were more pronounced in the C3D-GN group, but the difference was not significant (Table 9).
Acute changes, such as crescent formation, mesangial proliferation, vascular wall thickening, and interstitial inflammation, seemed more pronounced in the C3D-GN group than in the C3ND-GN group. Interstitial fibrosis, considered a chronic change, was significantly more frequent in the C3D-GN group.
Discussion
Our study revealed a significant prevalence of C3-dominant deposition in primary GN cases with differences in clinical and histological findings compared to cases of nondominant C3 deposition. We defined C3D-GN as C3 accumulation at least two grades greater than any other immune-reactant deposition as determined by IF, as previously proposed by Hou et al. [9]. That definition was used to reclassify C3G according to the degree of deposition in primary GN. However, as no evaluation such as pathological slide review or AP system analysis was performed, it was deemed more appropriate to record such cases as C3D-GNrather than C3G. Hou et al. [9] tested several IF criteria with varying stringencies for a more precise C3G definition, proposing that a strict definition, such as “C3 only,” is impractical. Thus, “C3 dominance and at least two grades more intense than any immune reactant (IgG, IgM, IgA, and C1q)” was proposed as a more useful classification. Accordingly, we established our definition of C3D-GN.
Based on our definition, cases of MPGN (61.3%) were most commonly reclassified as C3D-GN, with IgAN (25.8%) and MN (12.9%) cases also often reclassified. MPGN exhibits similar LM findings to those in C3G. Accordingly, our data indicate that C3D-GN occurred mostly in MPGN cases.
In our study comparing C3D-GN and C3ND-GN, LM findings revealed significantly more frequent severe mesangial proliferation in C3D-GN. Also, interstitial fibrosis, inflammation, and tubular atrophy were more progressive in C3D-GN; in particular, interstitial fibrosis was significantly more severe, in agreement with the histological findings of C3G [8]. However, these findings should be interpreted with caution as both acute inflammation and chronicity are more advanced in C3D-GN [10].
Although the C3D-GN definition is different from the strict C3G definition, C3-dominant deposition could be associated with AP abnormalities. AP dysregulation is central to the pathogenesis of C3G, which is related to genetic deficiencies, such as those of complement factor H (CFH), CFHR1-5, complement factor I, and CD46, or to auto-antibodies against factor H, factor B, and C3 convertase [4,7,9,11]. A strict definition of C3G with no or scarce deposition of immune factors derived from the classic pathway has been extensively adopted. However, if C3G is considered part of the disease process, deposition of other mediators may also occur during C3G [4].
IF analysis of C3D-GN revealed multiple immune-reactant depositions. C3 deposition was more prominent than that of other mediators, yet C1q, IgG, IgM, and IgA depositions were observed up to grade 1. C1q often initiates the classic pathway, and it can interfere with the AP by binding to C3b. Activation of CP via C1q can occur through deposits of IgG1 or IgG3, which have been reported to precede IgG4 deposition early in MN [12–14]. IgM staining could be attributed to nonspecific trapping in areas of sclerosis or capillary wall thickening [9]. IgA was deposited up to grade 1, as observed in cases where IgAN or MPGN was the primary diagnosis. In IgAN, IgA deposition may be attributed to the nature of the disease. With regard to MPGN, a review of the biopsy specimens from some cases revealed IgA deposition in previously diagnosed MPGN. Furthermore, in rare cases, IgA deposition was more dominant than that of other immune reactants [15,16]. Taken together, the findings indicate that other forms of immunologic injury may occur in the glomeruli under C3G, and immune reactions, such as CP activation, that induce the deposition of IgM, IgG, and other mediators cannot be excluded. In addition to MPGN, C3 may be deposited in IgAN or MN. When C3 deposition was accompanied by IgAN and MN, the renal outcome was worse in cases with above-moderate C3 dominance [17–19]. Therefore, it is necessary to review and reclassify the primary diagnosis; due to practical problems, this should be considered a limitation of the present study.
The serum C3 level in the C3D-GN group was within normal limits but lower than that of the C3ND-GN group. Increased renal injury rates and a greater tendency for elevated C-reactive protein level were observed in the C3D-GN group. These observations may be a result of AP system dysregulation. Some previous studies have documented a decrease in renal function in C3G patients at diagnosis [20,21]. Considering these findings together with our current results, we hypothesize that a similar clinical pattern would be present as a result of AP dysregulation—that is, renal injury and inflammation would be attributed to damage caused by the already advanced CP activation as well as additional damage due to AP dysregulation. Regardless of which pathway is activated first, the amplification loop of complement activation can occur through a cascade that primarily involves proteins of the AP, with AP activation stimulating other complement systems to induce further tissue inflammation [22]. As our study did not evaluate AP dysregulation, we cannot conclude whether this was the driving mechanism. Nevertheless, we consider the possibility of AP dysregulation contributing to the observed findings.
With regard to clinical outcome, in the C3D-GN group, more patients progressed to ESRD, but this difference was not significant. At the time of diagnosis, acute injury was more prevalent, mesangial proliferation and interstitial inflammation indicating acute changes in pathological findings were severe, and interstitial fibrosis indicating chronicity showed significant progress in C3D-GN patients, which suggests that ESRD patients tended to be more prevalent in the C3D-GN group. However, the small number of patients likely limited our results. In addition, ESRD progression is influenced by the patient’s response to medications and other treatments and the presence of other diseases. However, this study did not evaluate those factors, so it is possible that they may have influenced the occurrence of ESRD. C3D-GN patients showed a higher rate of all-cause mortality, but the difference was not significant. In terms of C3G and mortality, a previous study documented lower survival with C3GN [20]. That study recorded fewer deaths and patients who died of sepsis and cancer and did not confirm how C3GN affected mortality. Other studies have determined that the mortality rate is high or better in C3G, although the relationship between C3G and mortality is uncertain [23,24]. However, a lower C3 level or the AP, which is assumed to be the main pathology in this study, has an effect on mortality and allowed hypothesis about the increase in mortality [25,26]. Nonsignificant differences may also have had an impact due to the small number of patients. The results of this study suggest the importance of reassessment of C3-dominant deposition if renal injury has progressed or acute inflammation is severe. In addition, the effects of APs in clinical manifestations can be considered if C3 is dominant in pathology and serum C3 level is low. If further studies show that dominant C3 deposition and lower C3 level are directly related to APs and if an acute-phase inflammatory marker or kidney injury is concurrent with a difference in C3 level, it is necessary to change or reclassify the diagnosis considering the treatment and prognosis of APs.
This study’s relevance lies in the reclassification of primary GN as C3-dominant and the evaluation of its clinical and histological characteristics. Studies on the classification and evaluation of primary GN based on C3 dominance are limited. By analyzing KoGNET data, we discovered that most GN cases in Republic of Korea were IgAN (34.17%), MN (9.17%), MCD (9.13%), and FSGS (7.65%), and all these conditions were more prevalent than MPGN (2.63%) [27]. As other primary GNs may also exhibit complement system abnormalities, we sought to determine the present aspects of C3 deposition.
The present study has some limitations. First, we reviewed IF data based on biopsy reports rather than through reevaluation of the original IF glass slides. Thus, the precise characteristics and distribution of staining for each factor could not be evaluated. Discrepancies in diagnosis and grading systems between hospitals can exist, so review of pathologic slides by a single pathologist is necessary. However, there were no data to identify patients, and it was difficult to retrieve and review all pathological slides in 18 centers. This is the major limitation of our study. Second, the primary diagnoses were not reviewed by a second pathologist. Such review might have resulted in different primary diagnosis through slide reinterpretation according to the complements and Ig deposition. If there was a change in the primary diagnosis after review, patient characteristics or outcomes may also have differed. However, as mentioned earlier, not conducting a slide review is also a limitation. In future research, we will perform a pathology slide review. Third, we did not evaluate AP dysregulation and could not determine whether it was an underlying cause of C3D-GN. If the AP system had been evaluated, the association with C3-dominant deposition and explanations of clinical and pathological differences may have been supplemented.
The current study showed that C3D-GN could constitute an additional category of primary GN. C3D-GN patients exhibited different pathologic and clinical features, highlighting the importance of considering complement deposition. With regard to C3-dominant deposition, additional studies are necessary to evaluate molecular and genetic abnormalities at each step and to assess AP system involvement. Taken together, the observed differences between C3-dominant and nondominant deposition in primary GN emphasize the importance of complement dysregulation in the pathophysiology of GN.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This research was funded by Seoul National University Bundang Hospital (grant number 14-2020-048).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: JR, HJC
Methodology: JR, JCJ
Software: EB, HES
Validation: SK, KYN
Formal analysis: JR, HES, JYR
Investigation: JR, SK
Data curation: EB, HES, SPK, SHK
Writing—original draft preparation: JR
Writing—review and editing: JR, HJC
Visualization: JCJ, JHJ, SK, SPK, SHK
Supervision: HJC, DWC
Project administration: TIC, BSC
All authors have read and agreed to the published version of the manuscript.