Patterns in renal diseases diagnosed by kidney biopsy: A single-center experience
Article information
Abstract
Background
The worldwide incidence of renal disease diagnosed by a kidney biopsy varies with age, race, sex, and region. Owing to a lack of studies and limited research resources for this disease in Korea, we investigated renal disease patterns by analyzing data from kidney biopsies performed over 13 years in a university-based teaching hospital in Korea.
Methods
Among 2,053 kidney biopsies performed from 2001 to 2013 at Kyungpook National University Hospital, 1,924 were retrospectively analyzed for histopathologic, demographic, and clinical data as well as laboratory results.
Results
Among the 1,924 studied kidney biopsies, 1,078 were males (56.0%) and the mean age was 37.7 ± 16.5 years. Asymptomatic urinary abnormalities were the most common clinical manifestation (62.5%). Immunoglobulin A nephropathy (IgAN) was the most common primary glomerular disease (37.4%), followed by minimal change disease (MCD), membranous nephropathy (MN), focal segmental glomerulonephritis and crescentic glomerulonephritis. Secondary glomerular diseases accounted for 10.3% of the total biopsies, with lupus nephritis being the most common (4.6%) followed by Henoch-Schönlein purpura nephritis and diabetic nephropathy. The most common cause of nephrotic syndrome was MCD (42.1%) followed by MN. Among patients seropositive for hepatitis B or C, IgAN (28.3% and 21.4%, respectively) was the most common cause.
Conclusion
IgAN and lupus nephritis were the most common primary and secondary glomerular diseases, respectively. Race, region, and practice patterns may affect renal disease patterns in different cohorts.
Introduction
The worldwide incidence of renal disease diagnosed by kidney biopsy differs by region, race, age, sex and clinical practice pattern. Although glomerular disease ranks third among causes of chronic kidney disease following diabetic nephropathy and hypertensive nephrosclerosis [1,2], it is clinically important because it can be reversed and often cured when detected and treated at an early stage.
A kidney biopsy is an essential diagnostic tool for accurate diagnosis of renal disease. It is not only useful for diagnosis but necessary for making effective treatment decisions and ascertaining the degree of active and chronic histologic change [3]. The degree of active or chronic change helps determine the prognosis and likelihood of response to treatment. Additionally, a kidney biopsy can be used to assess genetic diseases.
The prevalence of glomerular disease varies by geography. For example, Asia, Australia, and southern Europe have a high prevalence of immunoglobulin A nephropathy (IgAN) (20% to 40%), whereas the United States (US) and United Kingdom (UK), as well as Canada, South America, and Africa, have a low prevalence (2% to 10%) of IgAN [4].
Additionally, the incidence of glomerular disease varies according to age. Previous studies have shown that membranous nephropathy (MN) is the most common cause of adult nephrotic syndrome, whereas minimal change disease (MCD) is the predominant cause of nephrotic syndrome in children [5]. In elderly patients, the relative proportion of crescentic glomerulonephritis (GN), MN, and focal segmental glomerulonephritis (FSGS) is higher than in younger patients [6,7]. Moreover, the prevalence of glomerular disease varies according to the period. A recent study showed an increased prevalence of IgAN but a decreased prevalence of MN in a Korean cohort [8], whereas the prevalence of FSGS has been increasing in the US, Brazil and India [9-13]. In a recent study conducted in the UK, a significantly increased proportion of cases of IgAN but a decreased proportion of MN and a constant proportion of FSGS cases were reported [11].
An epidemiologic study of renal disease provides valuable information with respect to differences in race and geographical distribution of kidney diseases. In Asia (Japan, Singapore, and Hong Kong), southern Europe, Australia, and Finland, IgAN is the most common primary glomerular disease [4,8,9]. In the Middle East, FSGS is the most frequent renal disease according to the Saudi Arabian Registry [10]. MN is the most common primary cause of nephrotic syndrome in a northern European Caucasian population [11].
The prevalence rates and characteristics of renal disease are diverse and depend on the registries where they reported. The disease appears to be affected by practice patterns, such as indication of kidney biopsy, or racial or environmental influences. In a Uruguay registry, when nephrotic syndrome was the most frequent indication of kidney biopsy, MN was the most frequent glomerular disease. However, the incidence of IgA tended to increase significantly with the number of kidney biopsies for asymptomatic urinary abnormality (AUA) [14]. Moreover, easy access to procedures for kidney biopsies may influence the increased rates of biopsies and subsequently affect the epidemiologic characteristics of renal disease [15]. In a study of biopsy-proven renal disease in Korea, IgAN increased from 22.0% in the 1990s to 36.5% in theith an increased indication of kidney biopsy for AUA. The same study found MCD was the most common primary glomerular disease (26.3% to 26.6%) in the mid-1990s, when nephrotic syndrome was a major indication of kidney biopsy [6,16,17].
The pattern of renal diseases from different cohorts in different regions is therefore worth close examination. This study investigates patterns in renal diseases from a single center in Korea and compares different cohorts.
Methods
We reviewed patterns in renal disease diagnosed by kidney biopsy at Kyungpook National University Hospital in Korea. The cases of 2,053 adult who underwent a kidney biopsy in the hospital from 2001 to 2013 were reviewed retrospectively. Second biopsies and patients aged < 15 years were excluded. Of the 2,053 cases, 1,924 were analyzed after excluding 58 cases of graft biopsy and 71 cases of inadequate biopsy or incomplete records.
Indications for a kidney biopsy included microscopic hematuria and/or persistent proteinuria, nephrotic syndrome, acute nephritic syndrome, unexplained acute kidney injury, and clinical suspicion of renal involvement in systemic diseases such as systemic lupus erythematosus. Percutaneous, ultrasound-guided core needle biopsies were performed on patients lying in a prone position. Two kidney biopsy core specimens were extracted. Adequacy of the specimen, as determined by the number of glomeruli, was confirmed by a pathologist. The specimens were divided into three pieces for light, immunofluorescent, and electron-microscopic study.
We recorded the date of the biopsy, pathology report, demographic data on the patients, and clinical data including urinalysis and other laboratory results, as well as the clinical pattern of presentation. This study was approved by the Institutional Review Board (IRB) of Kyungpook National University Hospital (IRB number: 2016-03-028), and the IRB waived the requirement for written informed consent. All procedures adhered to the Declaration of Helsinki.
Glomerular disease was pathologically classified using World Health Organization recommendations [18]. Primary glomerular disease was classified into seven groups: MCD, IgAN, MN, FSGS, membranoproliferative glomerulonephritis (MPGN), crescentic GN (not fulfilling the criteria for systemic disease), and others. Secondary glomerular disease was classified into five groups: lupus nephritis, Henoch-Schönlein purpura (HSP) nephritis, diabetic nephropathy, anti-neutrophil cytoplasmic antibody-associated vasculitis (microscopic polyangiitis, Wegener’s granulomatosis, and Churg-Strauss syndrome) and others. Hereditary nephritis included thin basement membrane disease (TBMD) and Alport syndrome.
The clinical presentations and laboratory findings at the time of kidney biopsy were: i) isolated microscopic hematuria was defined as a urine red blood cell count ≥ 3 per high-power field and a 24-hour urine protein level < 30 mg/d; ii) AUA was defined as subnephrotic proteinuria and/or hematuria with no clinical symptoms or signs; iii) nephrotic syndrome was defined as a proteinuria level of > 3.5 g/d and a serum albumin level of < 2.5 g/dL; and iv) renal insufficiency was defined as an estimated glomerular filtration rate (eGFR) of < 60 mL/min/1.73 m2.
Data are presented as mean ± standard deviation or median (interquartile range) as appropriate. Ages and laboratory findings are presented as values at the time of kidney biopsy. Descriptive statistics were used for the analysis. All statistical analyses were performed with PASW Statistics for Windows version 18.0 (SPSS Inc., Chicago, IL, USA).
Results
Of the 1,924 kidney biopsies reviewed, 1,078 (56.0%) were males and 846 (44.0%) were females. The average age of total patients was 37.7 ± 16.5 years. Mean serum creatinine at the time of biopsy was 1.2 ± 1.6 mg/dL, blood urea nitrogen was 18.1 ± 12.8 mg/dL, and mean eGFR using the modification of diet in renal disease formula was 110.2 ± 64.6 mL/min/1.73 m2. The patients who had renal insufficiency with an eGFR < 60 mL/min/1.73 m2 numbered 344 (17.9%) (Table 1).
Pathologic distribution of renal diseases diagnosed via a kidney biopsy is presented in Table 2. Primary glomerular disease constituted 65.1% of the total number of reviewed cases. IgAN was the most common primary glomerular disease (37.4%), followed by MCD (8.8%), MN (7.6%), FSGS (6.8%), crescentic GN (2.3%) and others (1.1%). Secondary glomerular disease constituted 10.3% of the total cases. Lupus nephritis was the most common secondary glomerular disease (4.6%), followed by HSP nephritis (2.0%), diabetic nephropathy (1.3%) and others (1.9%). Hereditary nephritis accounted for 19.9% of the total cases, of which TBMD was the most common (19.6%), while tubulointerstitial nephritis accounted for 0.4% of the total.
The prevalence of primary glomerular disease differed by age at the time of presentation (Fig. 1). IgAN was the most common primary glomerular disease in patients aged < 60 years, whereas MN was the most common in patients aged > 60 years. At an age of ≥ 40 years, the proportion of MN increased. Interestingly, the proportion of crescentic GN was the second highest in those aged > 70 years.
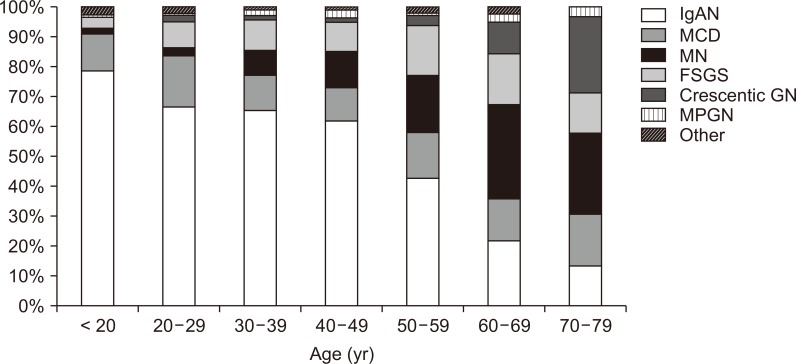
Distribution of primary glomerular disease by age.
FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; IgAN, immunoglobulin A nephropathy; MCD, minimal change disease; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis.
Elderly patients aged ≥ 65 years accounted for 155 (8.1%) of the total cases. MN (23.2%) was the most common primary glomerular disease, whereas HSP nephritis (5.2%) was the most common secondary glomerular disease in patients aged ≥ 65 years (Table 3).
When we analyzed renal disease based on the sex ratio, glomerular disease cases showed a male predominance, with MPGN showing a male predominance with a ratio of 2.3:1 (male:female) in primary glomerular disease, whereas diabetic nephropathy showing a ratio of 3.2:1 in secondary glomerular disease (Fig. 2).
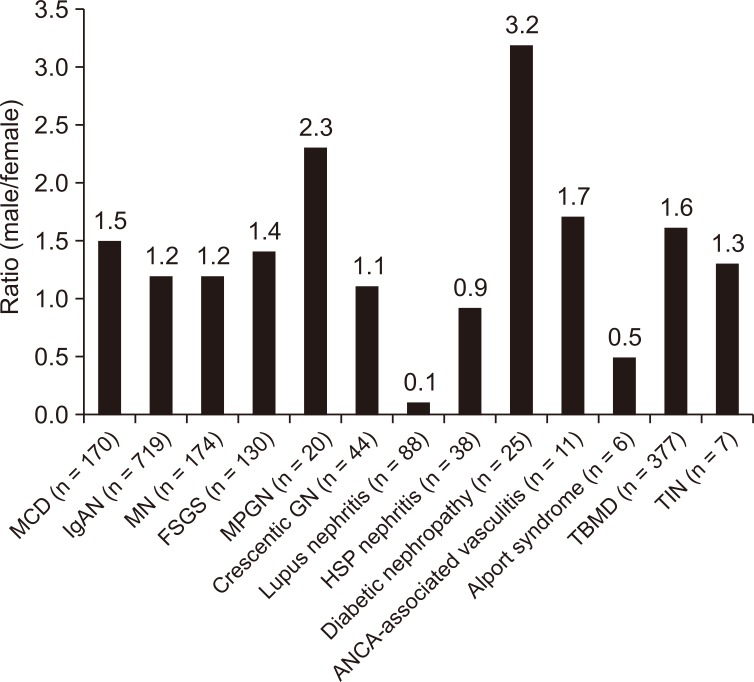
Distribution of renal disease by sex. The bar graph presents the ratio of males relative to females. Numbers are expressed as a ratio with the female value represented by 1.
ANCA, anti-neutrophil cytoplasmic antibody; FSGS, focal segmental glomerulosclerosis; GN, glomerulonephritis; HSP, Henoch-Schönlein purpura; IgAN, immunoglobulin A nephropathy; MCD, minimal change disease; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; TBMD, thin basement membrane disease; TIN, tubulointerstitial nephritis.
The clinical presentations at the time of biopsy were as follows: isolated microscopic hematuria in 246 cases (12.8%), AUA in 1,203 cases (62.5%), nephrotic syndrome in 145 cases (7.5%), and renal insufficiency in 344 cases (17.9%) (Table 4). Some patients presented with more than one clinical feature. For example, isolated microscopic hematuria with renal insufficiency or nephrotic syndrome with renal insufficiency was assigned to both groups.

Clinical presentations at the time of renal biopsy, n = 1,924 (1,938 cases), expressed as a percentage of all renal biopsies
Among the patients with isolated microscopic hematuria, TBMD was the most commonly diagnosed cause (53.2%) followed by IgAN (29.6%), MN (6.0%), MCD (2.0%), and HSP nephritis (2.0%) (Fig. 3A). One patient diagnosed with diabetic nephropathy presented with isolated microscopic hematuria. IgAN was the main cause of AUA (46.7%), followed by TBMD (26.1%), MCD (5.4%), MN (4.2%), and FSGS (4.2%) (Fig. 3B). Of the cases of nephrotic syndrome, MCD was the most common (42.1%), followed by MN (21.4%), FSGS (11.0%), lupus nephritis (10.3%), and HSP nephritis (3.4%) (Fig. 3C). When limited to primary glomerular disease, MCD was the most common (52.1%), followed by MN (26.4%), FSGS (13.6%), MPGN (3.4%), IgAN (2.5%), and crescentic GN (1.7%). Renal insufficiency was observed in 344 patients at the time of renal biopsy, and IgAN was found to be the most common (27.9%), followed by FSGS (18.8%), MCD (11.6%), crescentic GN (11.0%), and MN (4.0%) (Fig. 3D).
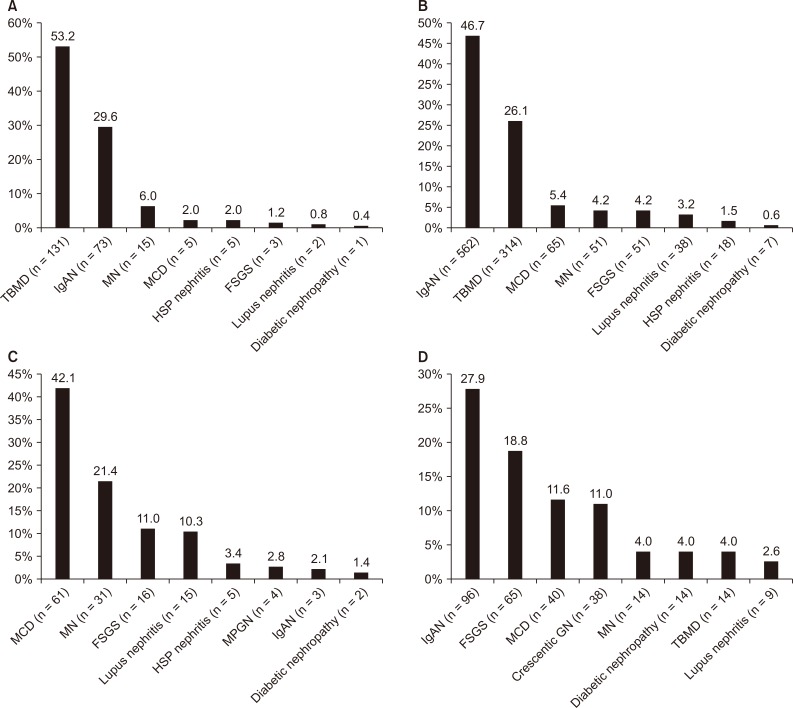
Distribution of renal disease by clinical presentation at the time of kidney biopsy.
(A) Patients with isolated microscopic hematuria (n = 246); (B) patients with asymptomatic urinary abnormality (n = 1,203); (C) patients with nephrotic syndrome (n = 145); (D) renal insufficiency (n = 344) with an eGFR < 60 mL/min/1.73 m2.
eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; HSP, Henoch-Schönlein purpura; IgAN, immunoglobulin A nephropathy; MCD, minimal change disease; MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis; TBMD, thin basement membrane disease.
Among the 60 patients with hepatitis B seropositivity, IgAN (28.3%) was the most common disease, followed by MN (21.7%), TBMD (16.7%), MCD (8.3%), FSGS (6.7%), MPGN (5.0%), and diabetic nephropathy (5.0%) (Table 5). IgAN was also the most common glomerular disease in hepatitis C seropositive patients, with MN, MCD, and MPGN following in the same proportion.
When glomerular diseases were analyzed at 4-year intervals, IgAN remained the most common cause across all periods. The relative frequency of FSGS increased from 5.4% in the first period (2001-2004) to 9.3% in the final period (2009-2013, P < 0.05). TBMD decreased significantly from 19.5% in the first period to 11.5% in the last period (P < 0.05), but the proportion of IgAN and MCD did not change (Fig. 4).
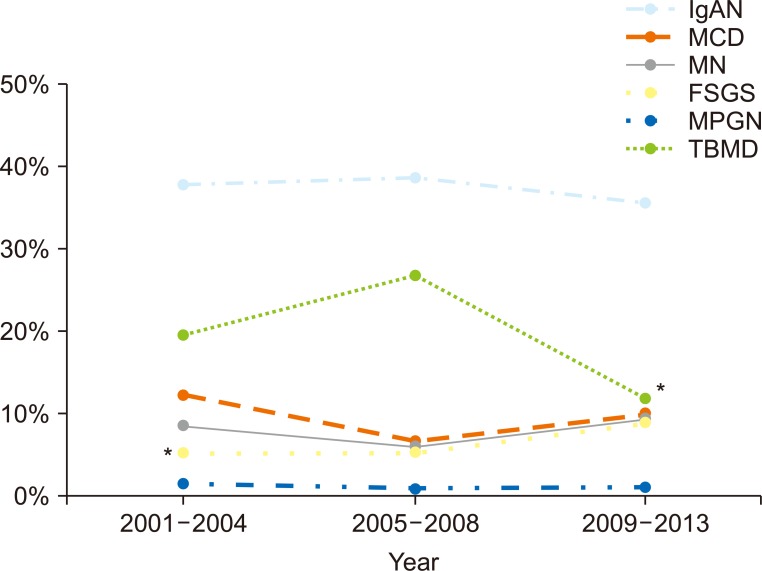
The distribution and percentage of each of the major glomerular diseases during 4-, 4-, and 5-year time intervals.
*P < 0.05, compared with two other time intervals. Immunoglobulin A nephropathy (IgAN) was the most common cause of glomerular disease across all periods. The relative frequency of focal segmental glomerulosclerosis (FSGS) increased from 5.4% in the first period (2001-2004) to 9.3% in the final period (2009-2013, P < 0.05). Thin basement membrane disease (TBMD) decreased significantly from 19.5% in the first period to 11.5% in the final period (P < 0.05). The percentage of IgAN and minimal change disease (MCD) did not show any trends.
MN, membranous nephropathy; MPGN, membranoproliferative glomerulonephritis.
Discussion
In this report from a single university-based teaching hospital in Korea, we obtained data from 1,924 patients with kidney disease who underwent kidney biopsies from 2001 to 2013.
In this descriptive result of kidney biopsy, we confirmed that IgAN was the most common primary glomerular disease (37.4%), whereas lupus nephritis was the most common secondary form (4.6%). Additionally, the incidence of primary glomerular disease differed by age. IgA nephropathy was the most prevalent in young patients, whereas MN was prevalent in the elderly.
Consistent with the findings in this study, IgAN has been reported to be the most common primary glomerular disease in East Asia (Japan, China, Singapore, and Hong Kong), Australia, North America, and some European countries, such as Italy, Spain, Hungary, France, the Netherlands, and the Czech Republic. However, it is rare in African-Americans, in whom FSGS is more common [5,12,13,19-21].
In Japan, 33% of patients with primary glomerular disease have IgAN [22]. The prevalence of IgAN was higher in China (45% to 50%) compared with 22% in the US and 17% to 37% in Europe [9]. Racial differences may be associated with geographic differences in the frequency of IgAN because Asians may be more susceptible and African-Americans may be less susceptible; this reflects the correlation of variation in IgAN susceptibility loci, including chromosome 6p21 and differences in disease prevalence [23].
Patients who underwent a kidney biopsy in our cohort were younger than those in other cohorts. In 2007-2008, the average age of the patients in the Japanese renal biopsy registry was 44.4 ± 21 years [22]. However, in this study, the mean age of the patients was 37.7 years, and the percentage of patients aged < 50 years was 60%; a selection bias may be involved that affected the results by showing a higher prevalence of IgAN.
In the elderly group, MN was the most common cause of primary glomerular disease in our patients. In a recent analysis of the Japanese renal biopsy registry, MN was the most common primary glomerular disease among the elderly [24], whereas amyloidosis, MN, and vasculitis associated with anti-neutrophil cytoplasmic antibodies were found to be dominant in other registries [25]. Amyloidosis was the most common form (16.9%) among the elderly according to the Spanish registry of GN [26], while MN was predominant (14.4%) in a retrospective analysis of 111 elderly patients conducted in South Africa [27].
Regarding secondary glomerular disease, lupus nephritis was the most common cause (4.5%) in our cohort. Consistently, the most frequent secondary glomerular disease was lupus nephritis in Korea (8.7%) [8], China (54.3%) [28], Hong Kong (20.5%) [29], Spain (8.8%) [30], Italy (2.6 per million population/year) [20,31], Brazil (9.8%) [32], Bahrain (15.7%) [33], and Australia (13.9%) [34].
A kidney biopsy should be performed to evaluate suspected idiopathic nephrotic syndrome in adults. We found that the most common cause of nephrotic syndrome was MCD (42.1%), followed by MN and FSGS. Similar trends have been reported in Japan [12] and Korea [8]. In European Caucasian populations, MN is the most common cause of nephrotic syndrome [5,11,31]. Although the different etiologies of nephrotic syndrome remain unknown, race, geography, and environmental factors may be responsible for the differences observed.
In our study, the sex ratio (male:female) of renal disease ranged from 0.1:1 to 3.2:1, and glomerular disease cases showed mostly male predominance. Previous studies have shown that glomerular disease is usually predominant in males or associated with an equivalent sex ratio in MCD, FSGS, MN, MPGN, IgAN, and HSP nephritis. Among the Korean population, the sex ratio for IgAN is similar; in our study, the sex ratio for IgAN was 1.2:1, which was similar to the results from other studies in Korea [8,16].
In a previous study, MN was most frequently reported in hepatitis B virus (HBV)-associated nephropathy in Asian populations [35]. In our study, the most common cause of glomerular disease in patients who tested positive for the hepatitis B surface antigen (HBsAg) was IgAN (28.3%), followed by MN (21.7%), TBMD (16.7%), MCD (8.3%), and FSGS (6.7%). The prevalence of HBV-associated nephropathy was closely related with the geographic patterns of HBV prevalence [36]. In South Korea, which is an intermediate endemic area for HBV, the prevalence of HBV seropositivity decreased from 8% to 10% in the 1980s and 1990s to 2% to 7% in 2017 owing to perinatal HBV vaccination [37]. Long-term studies may help elucidate whether a reduction in HBV seropositivity affects the glomerular disease pattern associated with HBV. In our cohort, the most common glomerular disease in patients with hepatitis C was also IgAN, unlike other studies, in which MPGN was the most frequent in the context of cryoglobulinemia [38].
With regard to epidemiological changes of poststreptococcal glomerulonephritis (PSGN), although the incidence has decreased from the 1970s to the 1990s and it is now rare in industrialized countries [39], PSGN remains common in communities with a low socioeconomic status and in patients with alcoholism, diabetes, or a history of intravenous drug abuse, all of which are associated with an immunocompromised condition or old age [40]. However, in this study, we could not observe the epidemiologic characteristics of PSGN due to the limited number of cases.
Although the time period for this study was shorter than that of previous studies conducted in Korea, TBMD in this study fell significantly from 19.5% in the first period (2001-2004) to 11.5% in the final period (2009-2013, P < 0.05).
Regarding changing patterns of renal disease, a report from Korea revealed the comparative effects of time period on the prevalence of glomerular disease. The relative frequency of IgAN increased (from 25.6% to 34.5%), while the relative frequency of MCD (from 23.2% to 7.0%) and MPGN (from 6.7% to 1.7%) decreased significantly during the past 20 years [8]. The changing pattern of renal diseases diagnosed by kidney biopsies was also considered to be influenced by the clinical practice patterns for kidney biopsies, such as indication of kidney biopsy. Decreased incidence of TBMD in this study seems to be attributed to the changing practice pattern for kidney biopsy indication. Compared with the previous period, when young patients with isolated microscopic hematuria were diagnosed at a routine health screening examination for military service, most received a kidney biopsy for the purpose of diagnosis. However, in the recent period, kidney biopsies were performed less frequently for the same indication at our institution. Moreover, the time period of this study was shorter than that of the previous Korean study.
This study has several limitations. It was not based on national registry data. Moreover, with the exception of TBMD, we could not identify a changing pattern for each glomerular disease over a 4-year time interval, owing to the fact that this study was based on only 13 years of data. This study also did not reflect the recent data. Finally, our study could not prove HBV-associated GN but simply described the frequency of glomerular disease in patients with HBsAg seropositivity because of the limited data on serum hepatitis B e antigen (HBeAg) and/or HBsAg or HBeAg immune complexes in kidney tissues. However, kidney biopsy data from a tertiary university-based hospital may provide representative information on Korean epidemiologic features of renal disease. An analysis of the changing patterns of renal diseases from longitudinal kidney biopsy data collected over a longer term would be useful.
In summary, from an investigation conducted on patterns in renal diseases diagnosed via kidney biopsy, we found that IgAN was the most common primary glomerular disease and lupus nephritis was the most common secondary glomerular disease. It is also meaningful to compare the pattern of renal diseases among different registries according to race, geographic region and clinical practice patterns.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Sun-Hee Park and Ji-Young Choi participated in proposal of idea and designing the study. Taehoon Yim, Sang-Un Kim, Sangmi Park, and Jeong-Hoon Lim participated in data acquisition. Sun-Hee Park, Ji-Young Choi, Taehoon Yim, Sang-Un Kim, Hee-Yeon Jung, and Jang-Hee Cho participated in data analysis and interpretation. Taehoon Yim, Sang-Un Kim participated in statistical analysis. Sun-Hee Park, Ji-Young Choi, Taehoon Yim, and Sang-Un Kim wrote the manuscript. Chan-Duck Kim, and Yong-Lim Kim participated in the review and supervision. Man-Hoon Han and Yong-Jin Kim reviewed the pathological slides and diagnosed kidney diseases as pathologist. All authors read and approved the final manuscript.




