Timing for initiation of sequential continuous renal replacement therapy in patients on extracorporeal membrane oxygenation
Article information
Abstract
Background
Extracorporeal membrane oxygenation (ECMO) is a lifesaving therapy used in critically ill patients with severe cardiopulmonary dysfunction. Continuous renal replacement therapy (CRRT) is supplemented to treat fluid overload, acute kidney injury, and electrolyte disturbances during ECMO. However, the best time to initiate CRRT is not well-defined. We performed this study to identify the optimal timing of CRRT for ECMO.
Methods
We conducted a multicenter retrospective cohort study of 296 patients over 12 years. Patients received CRRT during ECMO at Seoul National University Hospital, Seoul National University Bundang Hospital, or Yonsei University Hospital. We assigned patients to an early or late CRRT group depending on the CRRT initiation time. We considered early CRRT to be CRRT instituted within 72 hours of ECMO initiation.
Results
Among 296 patients, 212 patients (71.6%) received early CRRT. After using a propensity score matching method, 47 patients were included in each group. The time from ECMO initiation to CRRT initiation was 1.1 ± 0.9 days in the early CRRT group and 14.6 ± 18.6 days in the late CRRT group. No difference in patients’ mortality (P = 0.834) or hospital stay (P = 0.627) between the early and late CRRT groups was found. After adjusting all covariables, there was no significant difference in mortality between the early and late CRRT groups (hazard ratio, 0.697; 95% confidence interval, 0.410–1.184; P = 0.182).
Conclusion
This study showed that early CRRT may not be superior to late CRRT in ECMO patients. Further clinical trials are warranted.
Introduction
Extracorporeal membrane oxygenation (ECMO) is a lifesaving therapy used in critically ill patients with severe cardiopulmonary dysfunction. This technique was first successfully used in 1972 in patients with acute post-traumatic respiratory failure [1]. At first, ECMO was primarily used for respiratory failure of newborns. But after the H1N1 influenza pandemic, several studies were conducted on ECMO for adults, and ECMO techniques have since improved [2–5].
Continuous renal replacement therapy (CRRT) is often applied to treat uremia, fluid overload, electrolyte disturbances, and acid-base abnormalities in critically ill patients. Positive volume balance and acute kidney injury (AKI) are well-recognized risk factors for mortality in patients receiving ECMO [6–9]. The most common causes for initiating renal replacement therapy in ECMO patients are fluid overload (43%), prevention of fluid overload (16%), AKI (35%), and electrolyte disturbances (4%) [10]. CRRT is supplemented to 40–60% of ECMO patients [7,11–13]. However, the best time to initiate CRRT is not well-defined. Early initiation of CRRT may be effective in managing fluid overload, AKI, and acid-base disturbances. On the other hand, late initiation of CRRT has the advantage of allowing time for spontaneous renal recovery. Although there are many studies comparing the early initiation of CRRT with the late initiation of CRRT in critically ill patients, there are few studies on the timing of CRRT in ECMO patients [12,13].
Thus, we conducted a multicenter retrospective cohort study to evaluate the risk of mortality associated with the timing of CRRT in ECMO patients. We hypothesized that early initiation of CRRT is associated with reduced mortality compared with late initiation of CRRT. To minimize confounding biases, we used a propensity score analysis.
Methods
Study design and patients
This retrospective study included adult patients 15 years or older. All patients underwent ECMO treatment at Seoul National University Bundang Hospital, Seoul National University Hospital, or Yonsei University Severance Hospital, all of which are tertiary referral hospitals. From January 2005 to May 2016, 838 patients received ECMO treatment. Patients were excluded if they did not receive CRRT (n = 383), if they had been receiving CRRT before initiating ECMO (n = 148), or if they received ECMO and CRRT at the same time (n = 11). Finally, 296 patients were enrolled in this study (Fig. 1).
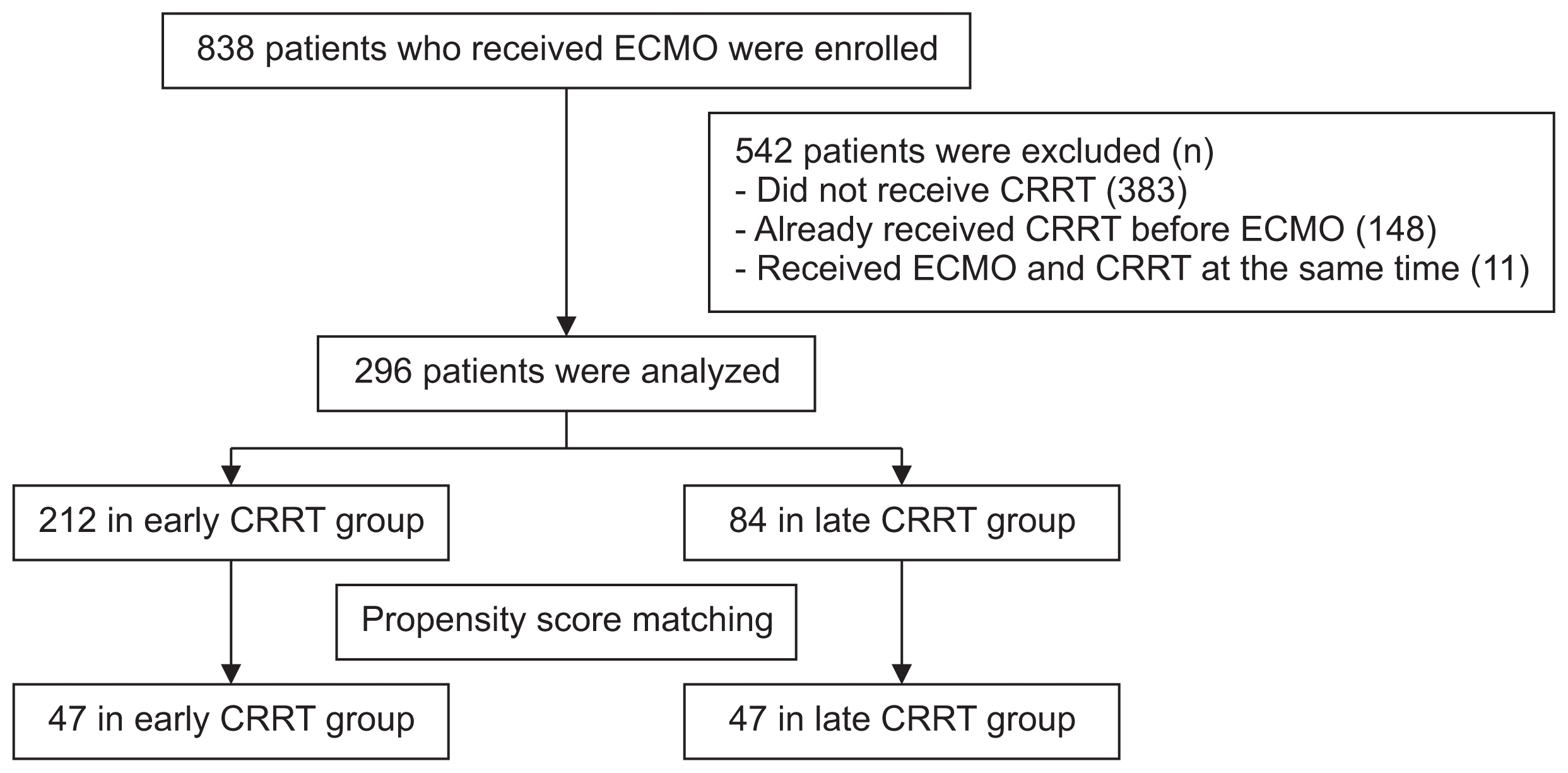
Flow chart of the study population
CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation.
This study was approved by the Institutional Review Board of Seoul National University Hospital (H1607-072-776), Seoul National University Bundang Hospital (B-1607/354-101), and Yonsei University Severance Hospital (4-2016-0867).
Measurements and definitions
We collected the following patient data from medical records: age, sex, body mass index (BMI), ECMO settings, intensive care unit days prior to ECMO, CRRT settings, CRRT duration, fluid balance, white blood cells, hemoglobin, platelet, C-reactive protein (CRP), albumin, total cholesterol, total bilirubin, blood urea nitrogen (BUN), creatinine (Cr), estimated glomerular filtration rate (eGFR), sodium, potassium, chloride, total CO2 (TCO2), and pH. Clinical and laboratory data were obtained within the 24-hour period prior to ECMO initiation. We assessed each patient’s history of comorbidities based on the International Classification of Disease, 10th revision (ICD 10), as follows; hypertension (I10–I15), diabetes mellitus (E10–E14), heart failure (I50), and chronic obstructive pulmonary disease (J40–J46). BMI was calculated using the following formula: BMI = body weight (kg) / height (m)2 [14]. Fluid balance was calculated as the difference between fluid in and out within 72 hours after ECMO initiation: fluid balance = (total fluid intake [mL] −total fluid output [mL]) / body weight (kg) [7]. We used the modification of diet in renal disease formula with serum Cr, age, and sex: eGFR (mL/min/1.73 m2) = 186 × serum creatinine (mg/dL)−1.154 × age (years)−0.203 × 0.742 (if female) [15].
We assigned patients to either the early or late CRRT group depending on the CRRT initiation time. We considered early CRRT to be CRRT added within 72 hours of ECMO initiation based on fluid balance [7,16]. The primary outcome was overall mortality within 30 days after ECMO initiation. Data from patients who survived 30 days after ECMO initiation were censored, and patient data lost to follow-up before 30 days after ECMO initiation were censored at the last follow-up date.
Statistical analysis
To reduce potential confounding biases in the study cohort, propensity score matching was applied. Propensity scores for each patient were calculated using a multivariate logistic regression analysis with the following covariates: age, sex, white blood cells, hemoglobin, platelets, albumin, sodium, potassium, BUN, Cr, eGFR, fluid balance, CRP, TCO2, and pH. We used a greedy 8-1-digit matching algorithm to match patients in a 1:1 ratio. Propensity score matching was conducted with SAS ver. 9.1.3 (SAS Institute, Cary, NC, USA).
Continuous variables were presented as the mean ± standard deviation, and categorical variables were presented as frequencies and percentages. The intergroup comparison of continuous variables was analyzed by Student’s t test. A chi-square test was used for intergroup comparisons of categorical variables. The survival curve was estimated using the Kaplan–Meier method, and the statistical significance was calculated using the log-rank test. The Cox proportional hazard regression model was used to evaluate the crude hazard ratio (HR) and propensity score-adjusted HR between mortality and the timing of CRRT in ECMO patients. A value of P < 0.05 was used to determine statistical significance. IBM SPSS Statistics version 20 (IBM Co., Armonk, NY, USA) was used for all data analyses except the propensity score matching method.
Results
A total of 296 patients were enrolled in the study, of which 212 were in the early CRRT group and 84 were in the late CRRT group. The mean age of the patients was 58.8 ± 16.6 years, and 194 (65.5%) were male. The mean time from ECMO initiation to receive CRRT was 0.8 ± 0.8 days in the early CRRT group and 17.4 ± 30.4 days in the late CRRT group. Among the participants, 210 (70.9%) and 86 (29.1%) received venoarterial (VA) and venovenous (VV) ECMO support, respectively. The reasons for ECMO support were non-operative cardiovascular causes (56.1%), non-adult respiratory distress syndrome (ARDS) lung causes (15.5%), ARDS (14.5%), post-cardiotomy (8.1%), and other causes (5.7%). The data showed that 118 (39.9%) patients underwent cardiopulmonary resuscitation (CPR) within the 24-hour period prior to ECMO initiation. Last, 188 (89.5%) patients with cardiogenic shock received VA ECMO, and 81 (94.2%) patients with respiratory failure received VV ECMO.
Clinical characteristics of the patients in this study are shown in Table 1. The late CRRT group received more VV ECMO support than the early CRRT group. Reasons for ECMO support were associated with the timing of CRRT initiation. CPR within the 24-hour period prior to ECMO initiation was higher in the early CRRT group than in the late CRRT group. Mortality was significantly lower in the late CRRT group than in the early CRRT group, and hospital stay was significantly shorter in the early CRRT group than in the late CRRT group. The late CRRT group had a higher CRP level than the early CRRT group. Serum Cr was significantly lower in the late CRRT group than in the early CRRT group, and eGFR was significantly higher in the late CRRT group than in the early CRRT group. The early CRRT group had significantly lower TCO2 and pH levels compared with the late CRRT group. After using a propensity score matching method, 47 patients were included in each cohort. The mean age was 60.5 ± 15.9 years, and 59 (62.8%) patients were male. The mean time from ECMO initiation to CRRT initiation was 1.1 ± 0.9 days in the early CRRT group and 14.6 ± 18.6 days in the late CRRT group. There were no statistical differences between the early and late CRRT groups in terms of ECMO mode, reasons for ECMO support, CPR within the 24-hour period prior to ECMO initiation, patient mortality, hospital stay, CRP, serum Cr, eGFR, TCO2, or pH.
Table 2 shows the results of the Cox proportional hazards analyses used to identify an association between the timing of CRRT and the overall mortality in ECMO patients. The early CRRT group had poorer survival than the late CRRT group, based on a univariate analysis (HR, 0.427; 95% confidence interval [CI], 0.304–0.601; P < 0.001) and a multivariate analysis (HR, 0.432; 95% CI, 0.295–0.632; P < 0.001) of the entire cohort. In the propensity-matched patients, however, the late CRRT group had no significant survival benefit compared to the early CRRT group, as assessed by crude analysis (HR, 0.708; 95% CI, 0.417–1.202; P = 0.201). After adjusting for all covariables, there was no significant association between the timing of CRRT and mortality (HR, 0.697; 95% CI, 0.410–1.184; P = 0.182).
The Kaplan–Meier survival analyses are shown in Fig. 2. For the entire cohort, the cumulative incidence of overall mortality was higher in the early CRRT group than in the late CRRT group (P < 0.001). However, there was no significant difference in overall mortality between the early CRRT group and the late CRRT group for the propensity-matched cohort (P = 0.199).
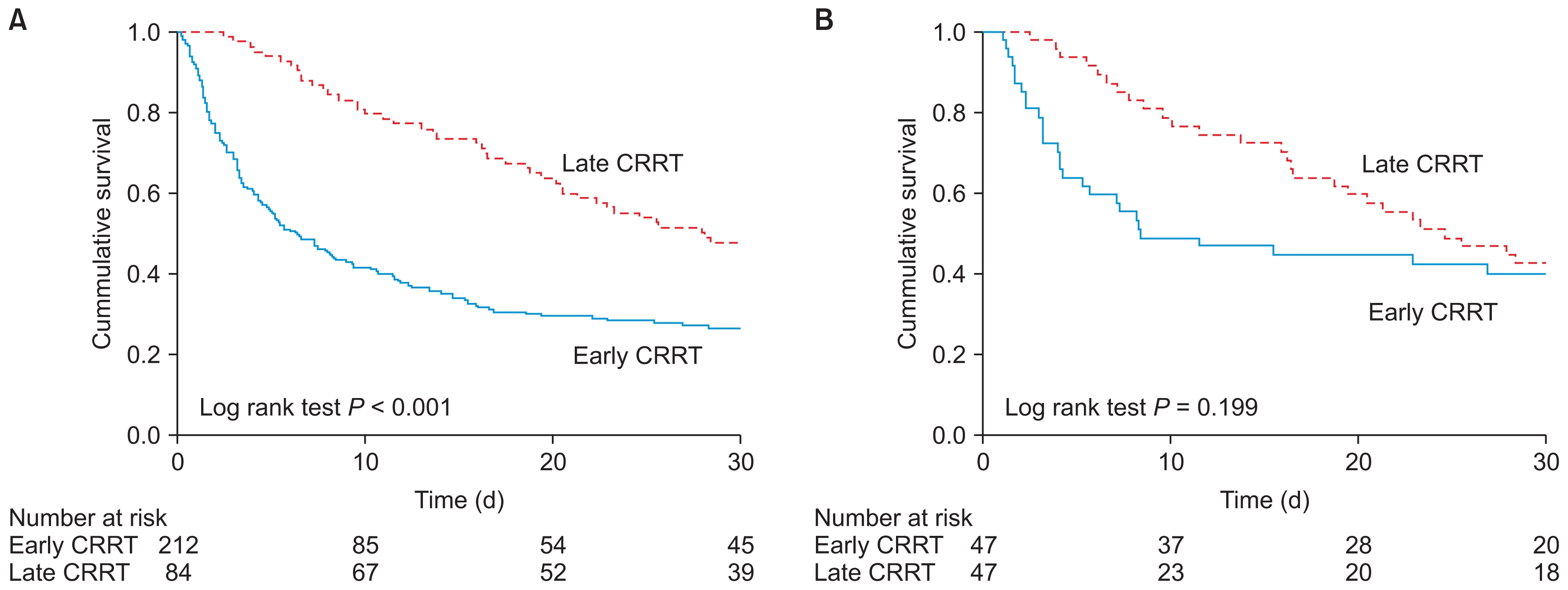
Kaplan–Meier survival according to continuous renal replacement therapy (CRRT) initiation among (A) all patients and (B) propensity-matched patients.
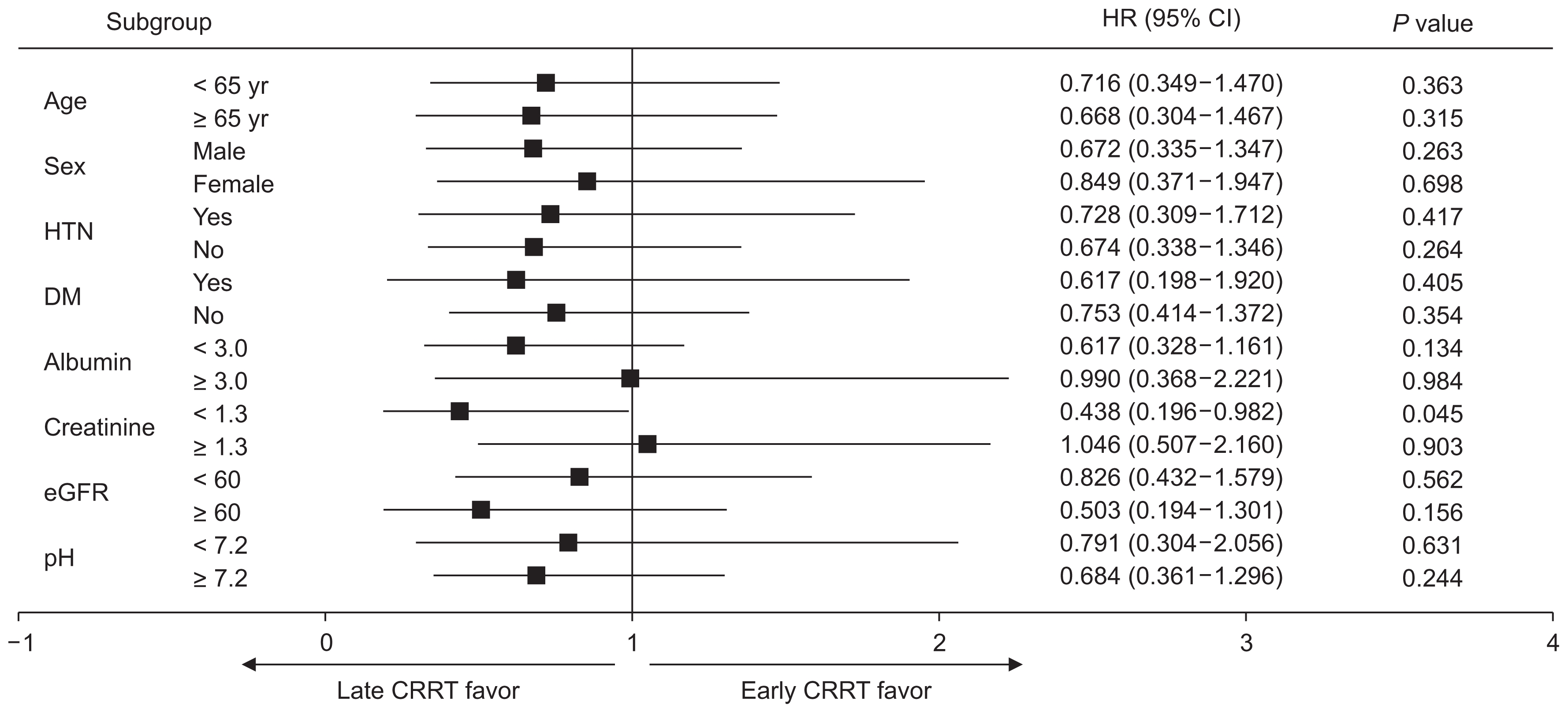
Fig. 3 shows the subgroup analyses of the estimated HR for the timing of CRRT and overall mortality in the propensity-matched patients. A higher mortality risk was observed in early-CRRT-group patients with Cr levels ≥ 1.3 mg/dL (HR, 1.046; 95% CI, 0.507–2.160; P = 0.903) than in late-CRRT-group patients with Cr levels < 1.3 mg/dL. Late-CRRT-group patients with Cr levels < 1.3 mg/dL showed better survival than early-CRRT-group patients with Cr levels < 1.3 mg/dL (HR, 0.438; 95% CI, 0.196–0.982; P = 0.045). However, when we conducted a comparison (using two HRs) between patients with Cr levels < 1.3 mg/dL and those with Cr levels ≥1.3 mg/dL, there was no significant difference between these two groups (P = 0.059).
Discussion
We performed a multicenter retrospective cohort study of 296 critically ill patients who received ECMO to investigate the risk of mortality associated with the timing of CRRT in ECMO patients. We found that the early CRRT group may not be superior to the late CRRT group in ECMO patients, and there was no significant difference in hospital stay between the early CRRT group and the late CRRT group.
The question of when to initiate CRRT in critically ill patients with AKI has long been a controversial concern for clinicians [17]. There is a consensus that CRRT should be initiated urgently following life-threatening complications of AKI, such as refractory volume overload, hyperkalemia, or metabolic acidosis. But there remains controversy over when to start CRRT in critically ill patients without such complications [18]. Recent data have shown that the traditional indications for initiating CRRT are less commonly encountered in critically ill patients (nor are they the most common triggers for the initiation of CRRT) [19,20]. In clinical practice, the decision to initiate CRRT varies. It is determined by physician beliefs and patient and organizational characteristics [21,22]. In addition, the definitions of “early” and “late” are relative. Previous studies have used serum urea nitrogen, serum creatinine, urine output, timing relative to intensive care unit admission, and timing relative to the development of traditional indications for initiating CRRT (such as fluid overload, hyperkalemia, and metabolic acidosis) as criteria to distinguish early CRRT from late CRRT [19,20,23]. This heterogeneity across studies in defining early and late CRRT has been a fundamental barrier to establishing definitive guidelines for the timing of CRRT initiation in critically ill patients. In the current study, we considered early CRRT to be CRRT added within 72 hours of ECMO initiation. We used propensity score matching to adjust prognostically important baseline characteristics. Several studies have revealed that fluid overload is the most common reason for initiating CRRT in ECMO patients, and fluid overload is significantly associated with mortality, impaired oxygenation, and longer length of hospital stay [7,10,24,25]. Fluid balance at day three is a particularly robust independent predictor for mortality in ECMO patients [7]. A recent randomized controlled trial (RCT) used volume status at 72 hours as the criterion for dividing the early and late CRRT groups in critically ill patients [16]. Thus, we chose 72 hours to distinguish between the early and late CRRT groups in ECMO patients.
In theory, the early initiation of CRRT has advantages for managing volume accumulation, acid-base disorders, electrolyte imbalances, and complications of uremia [26,27]. Despite the controversy, the early initiation of CRRT may confer benefits through modulating immune function and removing inflammatory mediators [28]. On the other hand, the late initiation of CRRT could allow time for spontaneous renal recovery, helping patients to avoid unnecessary CRRT [16,29]. Such patients may be at risk for complications of vascular access (such as bleeding or bacteremia) or complications of CRRT (including intradialytic hypotension, subtherapeutic levels of essential medications, and hypersensitivity to the extracorporeal circuit). Many observational studies and meta-analyses have demonstrated that the early initiation of CRRT in critically ill patients shows better patient survival than the late initiation of CRRT [27,30,31]. However, a meta-analysis of RCTs revealed that there was no significant difference in mortality between early and late CRRT groups [32]. Specifically, three RCTs showed that early initiation of CRRT had a beneficial effect on patient survival, but six RCTs showed that early initiation of CRRT did not improve patient survival (compared to late initiation of CRRT). The same analysis revealed there was no significant difference between early and late CRRT groups regarding intensive care unit length of stay, hospital stay, renal function recovery, CRRT dependence, duration of CRRT, renal recovery time, or mechanical ventilation time. More recently, two well-designed RCTs (Effect of Early vs. Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients with Acute Kidney Injury [ELAIN] and Artificial Kidney Initiation in Kidney Injury [AKIKI] trials) yielded conflicting results. The ELAIN trial was a single-center RCT of 231 critically ill patients in Germany [26]. Patients with stage 2 AKI (by Kidney Disease: Improving Global Outcomes [KDIGO] standards) or with plasma neutrophil gelatin-ase-associated lipocalin > 150 ng/mL were enrolled. Early CRRT was defined as initiating CRRT within 8 hours of a diagnosis of KDIGO stage 2 AKI, and delayed CRRT was defined as initiating CRRT within 12 hours of any of the following: KDIGO stage 3 AKI, BUN > 100 mg/dL, serum potassium > 6 mmol/L (and/or electrocardiography abnormalities), serum magnesium > 4 mmol/L, anuria, or organ edema resistant to diuretic treatment. The ELAIN trial revealed that early initiation of CRRT significantly reduced 90-day mortality. The AKIKI trial, a multicenter RCT of 620 patients in intensive care units in France, yielded different results [16]. The AKIKI trial enrolled patients with KDIGO stage 3 AKI who required mechanical ventilation, catecholamine infusion, or both, and did not have a potentially life-threatening complication directly related to renal failure. Early CRRT was defined as initiating CRRT immediately after randomization, and delayed CRRT was defined as initiating CRRT with at least one of the following criteria: BUN > 112 mg/dL, serum potassium > 6 mmol/L, pH < 7.15, acute pulmonary edema, or oliguria for more than 72 hours after randomization. This study demonstrated that mortality at day 60 did not differ significantly between the early and delayed groups.
In patients with ECMO, AKI and receiving CRRT are independent risk factors for mortality [8,33,34]. Thus, prevention of AKI and optimization of CRRT can improve patient survival. However, there have been very few studies on the timing of CRRT in ECMO patients [12,13]. In a single-center study of 153 pediatric cardiac patients receiving ECMO, the early CRRT group showed increased mortality [13]. Early CRRT was defined as initiating CRRT within 48 hours of ECMO initiation. The study compared the early CRRT group with an ECMO-only group. A total of 59 patients were included in the early CRRT group, and 94 patients belonged to the ECMO-only group. Patients in the early CRRT group had a higher mortality (P < 0.001). In a multivariate logistic regression analysis, patients with early CRRT initiation had a tripled risk of mortality. However, the study did not compare the early CRRT group with a late CRRT group. Furthermore, a single-center study of 200 adult patients undergoing ECMO showed opposite results [12]. Although that study was not aimed at the timing of CRRT in ECMO patients, it revealed that patients requiring late CRRT (relative to the time of ECMO insertion) had a higher risk of death. Both studies failed to consider fluid balance as a confounder. Our study included fluid balance as a confounder, and is the first to investigate the onset of CRRT by dividing patients into early and late CRRT groups.
To the best of our knowledge, this is the largest study to date to evaluate the risk of mortality associated with the timing of CRRT in ECMO patients. It is also the first study of CRRT and ECMO conducted using an Asian population. In our study of the entire cohort, the late CRRT group had better survival than the early CRRT group. Serum Cr was significantly higher in the early CRRT group than in the late CRRT group, and eGFR, TCO2, and pH were significantly lower in the early CRRT group than the late CRRT group. This finding implies that the early CRRT group had more severe illness, and these differences are related to an increased risk of mortality. Thus, we used propensity score matching to minimize confounding biases. After using the propensity score matching method, there was no significant difference in mortality or hospital stay between the groups. These results are consistent with the results of a recent RCT and a meta-analysis of RCTs [16,32].
Our study had some limitations. First, CRRT was not randomly administered to patients. Although propensity score matching was performed, this was a retrospective study. Several factors influencing outcomes were not fully collected, and the results could be subject to unmeasured confounders. Second, the definitions of early and late are arbitrary. We chose 72 hours to distinguish early CRRT from late CRRT, based on fluid balance. However, an earlier study selected 48 hours [13]. Furthermore, the time from ECMO support to initiation of CRRT varies widely. Third, there was a relatively small sample size in each group after propensity score matching. We included as many covariates as possible in relation to the initiation of CRRT for calculating the propensity score. This reduced each sample size after propensity score matching. Nevertheless, this is the largest study (and first multicenter analysis) to explore the timing of CRRT initiation in ECMO patients. Fourth, we did not have follow-up data, and therefore could not evaluate renal recovery and the effects of CRRT (such as correction of uremia, electrolyte disturbance, and metabolic acidosis).
In conclusion, our study demonstrated that early CRRT may not be superior to late CRRT in ECMO patients. CRRT may be delayed until more classic indications for CRRT are present. Further RCTs are needed to evaluate the effects of timing strategies on outcomes in ECMO patients.
Acknowledgments
This research was supported by the a grand of the KoreaHealth Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI17C1827).
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.