Risk of cancer in pre-dialysis chronic kidney disease: A nationwide population-based study with a matched control group
Article information
Abstract
Background
Cancer risk and epidemiology in pre-dialysis chronic kidney disease (CKD) warrant further investigation in a large-scale cohort.
Methods
We performed a nationwide population-based study using the national health insurance database of Korea. We screened records from 18,936,885 individuals who received a national health examination ≥ 2 times from 2009 to 2016. Pre-dialysis CKD was identified based on serum creatinine and dipstick albuminuria results. Individuals with preexisting cancer history, renal replacement therapy, or transient CKD were excluded. A control group without evidence of kidney function impairment and matched for age, sex, low-income status, and smoking history was included. Risk of cancers, as identified in the claims database, was investigated using a multivariable Cox regression model including matched variables and other unmatched clinical characteristics as covariates.
Results
A total of 471,758 people with pre-dialysis CKD and the same number of matched controls were included. Urinary (adjusted hazard ratio [HR], 1.97; 95% confidence interval [95% CI], 1.82–2.13) and hematopoietic (adjusted HR, 1.53; 95% CI, 1.38–1.68) malignancy risk was increased in pre-dialysis CKD and all CKD stages. However, the risk of digestive cancer was lower in the pre-dialysis CKD group (adjusted HR, 0.89; 95% CI, 0.87–0.92). The risk of digestive, respiratory, thyroid, and prostate malignancy demonstrated a non-linear association with CKD stage, with stage 1 or stage 4/5 CKD without dialysis demonstrating relatively lower risk.
Conclusion
Cancer risk varied in pre-dialysis CKD compared to controls, and the association between cancer risk and CKD stage varied depending on the cancer type.
Introduction
Cancer is a leading cause of death worldwide [1]. Importantly, cancer is involved in the majority of non-cardiovascular deaths in people with chronic kidney disease (CKD) [2]. As both CKD and malignancy increase with global aging, cancer in CKD patients will continue to become more clinically important [3,4].
Because early diagnosis of malignancy is a crucial factor that improves prognosis, understanding the epidemiologic distribution of cancer is particularly important. Several studies in dialysis-dependent patients or kidney transplant recipients identified that specific malignancies, such as urinary tract neoplasms, are highly prevalent in patients with impaired kidney function [5–10]. In addition, studies that investigated the risk of cancer in CKD patients without renal replacement therapy (RRT) found increased cancer-specific incidence or mortality in pre-dialysis CKD [11–15]. Nevertheless, a larger population-based study is warranted as previous studies included limited sample sizes of individuals with laboratory confirmed kidney dysfunction [11,12]. Results from a larger study could guide healthcare providers regarding screening for malignancy in the globally growing population of individuals with mild to moderate renal dysfunction [3,4]. However, it has been difficult to perform studies that include a sufficient number of pre-dialysis CKD patients due to the lack of longitudinal measurements of kidney function in most nationwide databases, which are needed to stratify CKD stages.
Here, we aimed to epidemiologically assess the type-specific cancer risk in a large cohort of pre-dialysis CKD by reviewing records from a national health screening program in which over 10 million people per year receive health examinations that include serum creatinine and dipstick albuminuria measurements [16]. We hypothesized that the degree of kidney dysfunction would be associated with site-specific cancer risk.
Methods
Ethical considerations
The Institutional Review Board of Seoul National University Hospital (IRB No. E-1801-027-913) approved the study. The usage of the National Health Insurance Database (NHID) was approved by the attending government organization. The study was conducted in accordance with the Declaration of Helsinki.
National Health Insurance Database and national general health screening in Korea
The NHID, provided by the National Health Insurance Service (NHIS) of Korea, is a database that includes a claims database and information on socio-demographic variables, national general health screening, and mortality [16]. With the national health screening program, over 10 million Korean people receive a health examination each year including serum creatinine and urinalysis albumin measurements at each screening [17]. This charge-free general health screening is provided for workplace subscribers and for every Korean over 40 years old at least biannually, and the overall examination rate has been over 70% since 2011. In addition, the NHIS applies unique insurance codes for those with a confirmed malignancy diagnosis, both for inpatient and outpatient care. The codes are required to receive additional coverage for cancer-related medical fees, resulting in a reliable method of cancer identification in the claims database.
Study population
Individuals who were screened ≥ 2 times between 2009 and 2016 using the kinetic Jaffe’s method for serum creatinine were included. We excluded 1) those with a previous history of cancer, 2) those receiving RRT at baseline (including both dialysis and kidney transplantation) or diagnosed for cancer after initiation of RRT, 3) those who were less than 19 years old, 4) those who had transient or fluctuating kidney function impairment (inconsistent albuminuria or reduced estimated glomerular filtration rate [eGFR, < 60 mL/min/1.73 m2]), and 5) those with missing information for the included variables. In the control group, those who had kidney disease related the 10th revision of the International Statistical Classification of Diseases and Related Health Problems (ICD-10) diagnostic codes (Supplementary Methods 1) were excluded [18].
Study groups
The CKD group included patients with pre-dialysis CKD, which we defined by the presence of consecutive laboratory evidence of CKD (e.g., dipstick albuminuria or eGFR < 60 mL/min/1.73 m2) for ≥ 2 sequential health screenings. The 1:1 matched control group was constructed from individuals without any CKD indicative laboratory results (albuminuria or reduced eGFR [< 60 mL/min/1.73 m2]) at each included health examination. Controls were matched based on age, sex, low-income status, and smoking history (none, previous, or current). We collected additional characteristics (e.g., history of diabetes, hypertension and body mass index) that were not used for matching but for which we adjusted in further analyses. Therefore, the control group was matched based on age, sex, and social factors in the general population rather than the presence significant comorbidities. The pre-dialysis CKD individuals were additionally categorized into the following groups according to baseline kidney function and dipstick albuminuria results from their first health examination: CKD stage 1, those who exhibited eGFR ≥ 90 mL/min/1.73 m2 and consecutive presence of a consecutive dipstick albuminuria; CKD stage 2, those with eGFR < 90 and ≥ 60 mL/min/1.73 m2 and presence of a consecutive dipstick albuminuria; CKD stage 3, those who exhibited eGFR < 60 and ≥ 30 mL/min/1.73 m2; and CKD stage 4/5, those who exhibited eGFR < 30 mL/min/1.73 m2 but who were not on RRT [19].
Data collection
The baseline characteristics collected from the NHID included age, sex, low-income status, history of smoking, and body mass index of the study subjects. Low-income status was defined as having an income lower than the nation’s 20th percentile. Serum creatinine data were collected from the examination records and we calculated the eGFR values using the Modification of Diet in Renal Disease (MDRD) method. History of underlying diabetes mellitus and hypertension was identified by the ICD-10 diagnostic codes and prescription history of relevant medications.
Study outcomes
The cancer risk was the main study outcome. Site-specific malignancy diagnoses were additionally reviewed for different body systems and organs using the ICD-10 diagnostic codes (Supplementary Method 1) [12]. The risk and incidence of cancer were also investigated for each CKD stage. Finally, cancer-associated mortalities included all-cause mortalities within 3 years of a cancer diagnosis because direct causes of death were not identified in the NHID.
Statistical analysis
Continuous variables are displayed as median (inter-quartile ranges) values. Categorical variables are displayed as numbers (percentages). Baseline differences among the study groups were investigated with the chi-squared test and the Kruskal–Wallis test. The differences in risk of each cancer were investigated using the multivariable Cox regression analysis with multiple adjustments, and the fully-adjusted model included both the matched variables (age, sex, low-income status, smoking history) and additional unmatched characteristics (history of hypertension, diabetes mellitus and body mass index). The association between underlying CKD and cancer-associated mortality for each type of cancer was also analyzed using the Cox regression analysis. The results from the fully-adjusted models are described in the text and figures otherwise significantly different trends were suspected between the models. We performed statistical analysis using the SAS ver. 9.4 program (SAS Institute, Cary, NC, USA) with two-sided P values < 0.05 considered statistically significant.
Results
Study population
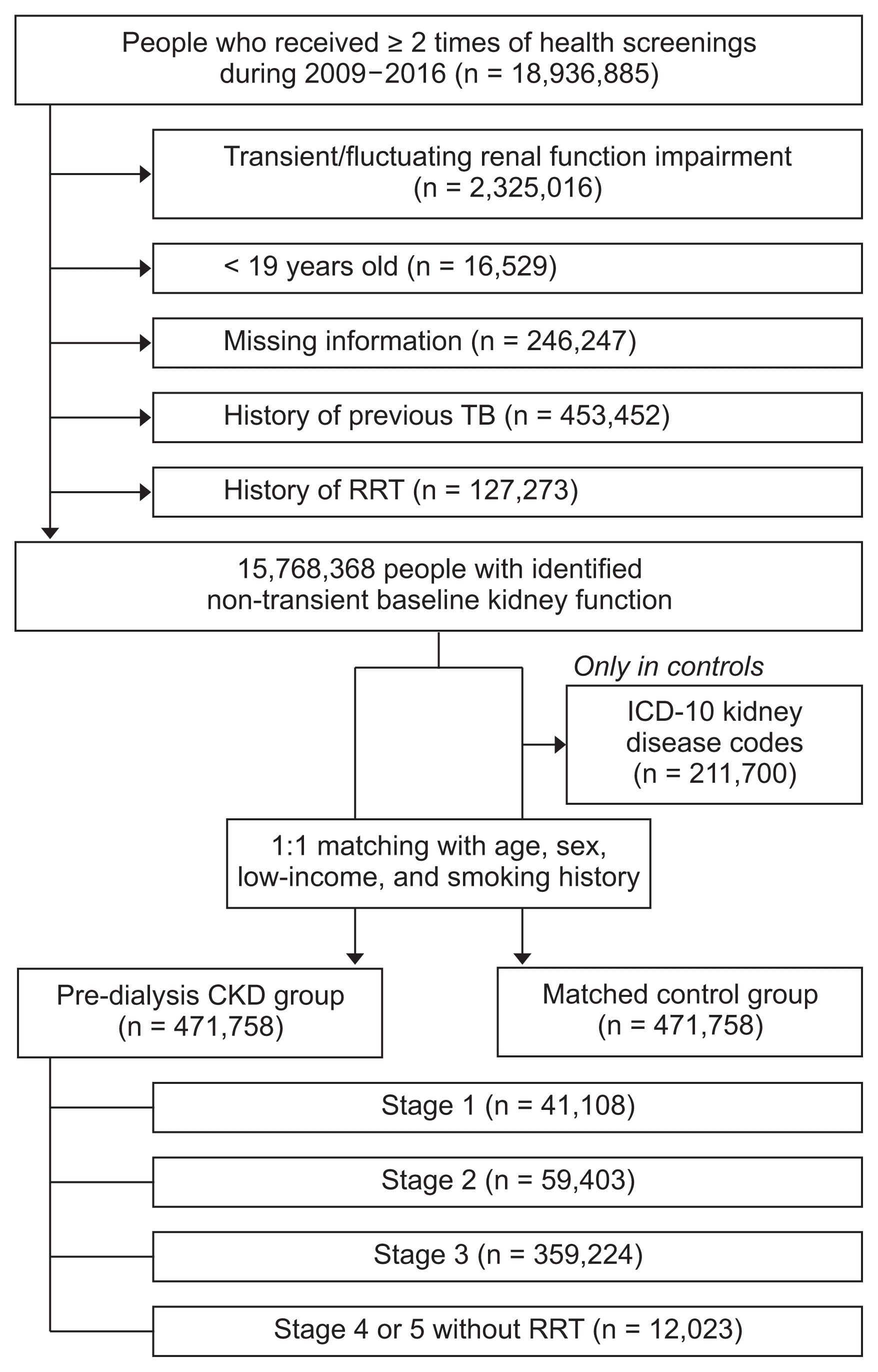
Total of 471,758 individuals with pre-dialysis CKD were included in the study, with the same number of individuals in the matched control group (Fig. 1). Within the CKD individuals, their CKD stages were stratified with 41,108 individuals exhibiting stage 1, 59,403 exhibiting CKD stage 2, 359,224 exhibiting CKD stage 3, and 12,023 exhibiting CKD stages 4/5 without RRT. The median follow-up duration was 4.77 years in the CKD group and 4.80 years in the matched control group.
Baseline characteristics
Due to the 1:1 matching, the variables included in the matching process had identical distributions between the CKD and the control groups (Table 1). The median age of the study population was 64 (55–71) years, and 51.5% were males. Regarding unmatched but adjusted variables, the CKD group exhibited more frequent hypertension and diabetes mellitus and exhibited a higher body weight and body mass index than the matched control group. When the CKD group was stratified according to their baseline kidney function (Supplementary Table 1), the higher stage CKD groups were older, included more males, and presented with more hypertension.
Risk of cancer in people with pre-dialysis CKD
The number of newly diagnosed malignancies and cancer incidences are presented in Table 2. The total cancer incidence was 1,019.76/100,000 person-years in the pre-dialysis CKD group, which was higher than 989.92/100,000 person-years in the matched control group. The digestive system had the largest cancer incidence, both in the pre-dialysis CKD group (445.18/100,000 person-years) and the matched control group (476.15/100,000 person-years). However, cancer incidence varied depending on the body system or organ affected. Based on regression analysis (Fig. 2 and Supplementary Table 2), the CKD group demonstrated increased risk of urinary tract and hematopoietic malignancies. In contrast, risk of stomach and thyroid cancers was decreased in the CKD group prior to adjustment for additional unmatched variables. After additionally adjusting for hypertension, diabetes and body mass index in our multivariable model, the risk of all digestive malignancies and stomach, colorectal and liver neoplasms was lower in the pre-dialysis CKD group than the controls. Testicular cancer risk was significantly increased in the CKD group only in the fully-adjusted model; however, the confidence interval was large due to the limited numbers of included events.
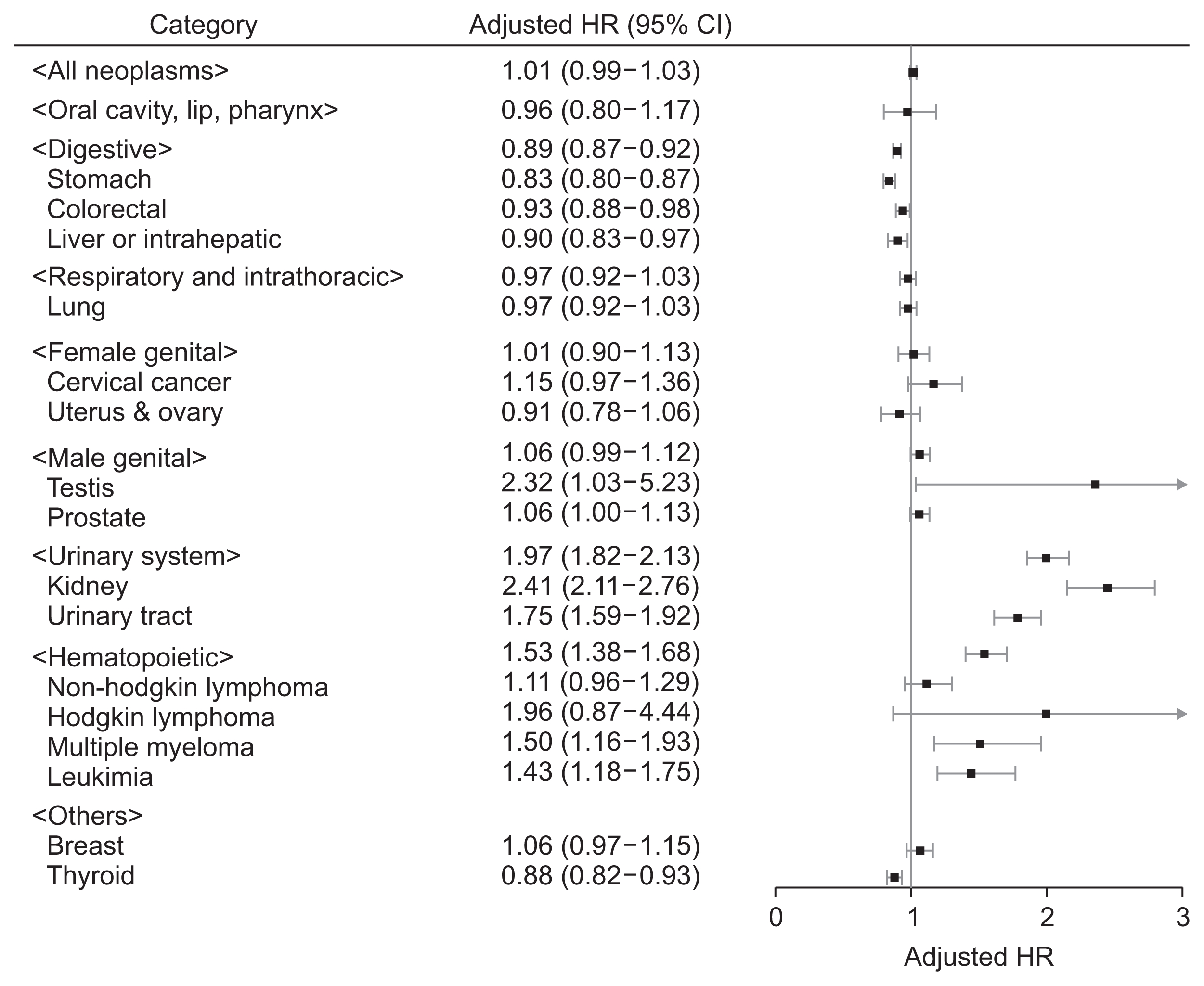
Forest plot presenting the cancer risk in the pre-dialysis chronic kidney disease group compared to the matched control group
The boxes indicate the hazard ratios (HRs), and the horizontal lines indicate the 95% confidence intervals (95% CIs). The adjusted HR were obtained from a fully-adjusted multivariable model that included the matched variables (age, sex, low-income status, and smoking history) and unmatched characteristics (history of hypertension, diabetes mellitus, and body mass index).
Risk of cancer according to CKD stage
The number of events and cancer incidences at each stage of CKD are presented in Supplementary Table 3. The risk of malignancies at each CKD stage demonstrated various trends in our regression analyses (Supplementary Table 4). Among the cancer categories assessed, those with risk that was significantly different from the matched control group are shown in Fig. 3. The urinary and hematopoietic system malignancy risk was significantly increased at every CKD stage; however, some subcategory risks did not reach significance in certain CKD stages. Meanwhile, the risk of gastrointestinal tract cancers, including stomach and colorectal malignancies, was significantly lower in people with CKD stage 1 or 4/5 without RRT. A similarly non-linear association was also observed for lung, prostate and thyroid cancers. The risk of liver cancer was significantly increased in CKD stage 2 but decreased in stage 3 or higher when compared to the matched control group.

Forest plot showing the cancer risk at each chronic kidney disease stage compared to the matched control group
Cancer types with representative differences are shown. The adjusted hazard ratios (HR) were obtained from a fully-adjusted multivariable model that included the matched variables (age, sex, low-income status, and smoking history) and unmatched characteristics (history of hypertension, diabetes mellitus and body mass index).
RRT, renal replacement therapy; 95% CI, 95% confidence interval.
Cancer-associated mortality in people with pre-dialysis CKD
Among those who developed malignancies, the 3-year mortality rate was 3,325/22,416 (14.8%) in the matched control group and 3,821/22,971 (16.6%) in the pre-dialysis CKD group. The risk of cancer-associated mortality was increased in people with baseline CKD in composite cancers and also in several malignancy categories (Table 3). However, colorectal, lung, liver, urinary system, cervix, thyroid, breast, and testis neoplasms and lymphomas did not demonstrate significantly increased mortality in individuals with CKD. Finally, people with baseline CKD had a lower risk of cancer-associated mortality following diagnosis for multiple myeloma or leukemia than the matched controls.
Cancer-associated mortality according to CKD stage
The 3-year mortality rate following a cancer diagnosis according to each baseline CKD stage is presented in Supplementary Table 5. The risk of cancer-associated mortality following diagnosis for a composite malignancy had a non-linear association with CKD stage, as the stage 1 and 4/5 without RRT groups demonstrated prominently increased risk of death. However, the statistical significance of this association was modest for each cancer type, and the statistical power varied. A similar non-linear association reached significance for digestive malignancies. Additionally, stage 4/5 CKD without RRT was associated with significantly increased risk of cancer-associated mortality for malignancies of the male genital system (prostate) and urinary system (kidney) and for colorectal cancers.
Supplementary materials are presented online (available at https://doi.org/10.23876/j.krcp.18.0131).
Discussion
Through this nationwide population-based study, we identified the type-specific cancer incidence in nearly half a million people with pre-dialysis CKD. When compared to the matched control group, the risk of urinary and hematopoietic system malignancies was higher in the pre-dialysis CKD population. Notable non-linear associations between CKD stage and the risk of several cancer types were observed. In addition, the presence of baseline CKD was associated with increased mortality after the development of malignancies in certain cancer categories.
The major strength of this study was the ability to assess cancer epidemiology and risk in one of the largest cohorts of individuals with pre-dialysis CKD confirmed by consecutive laboratory measurements. Increased risk of malignancies has been well established in patients with end-stage renal disease or after renal transplantation [5,7,10,20]. Several studies also suggested that the risk of cancer was elevated in pre-dialysis CKD patients, and lower eGFR values were reported to be related to a higher risk of cancer [11,13,14,20]. However, the limited numbers of people with confirmed kidney function impairment in these previous studies confined their interpretation [11–13,20]. Our study included the largest number of pre-dialysis CKD patients, and with this advantage we were able to report the cancer incidences of each malignancy category in pre-dialysis CKD. Therefore, these results could guide healthcare providers when evaluating malignancy risk in the growing number of individuals with pre-dialysis CKD.
Overall incidence of malignancies in the CKD population, which reached over 1,000/100,000 person-years, was much higher than reported incidences in the general population [21–23]. The type-specific incidences were also higher than the general population, and this might be related to the increased age of the CKD group. Therefore, clinicians should consider appropriate cancer screenings based on age in pre-dialysis CKD patients. In addition, considering the globally increasing number of individuals with CKD and the increasing age of the population, the importance of potential malignancy will continue to grow in people with renal function impairment [1].
Risk of cancer in pre-dialysis CKD patients, when compared to the matched control group, varied greatly by cancer types. As previous studies have reported, pre-dialysis CKD patients exhibited a prominently higher risk of urinary system or hematopoietic malignancies [11,13]. Unexpectedly, individuals with kidney function impairment had relatively lowered risk of thyroid and digestive malignancies compared to matched controls. The risk of stomach cancer was also lower in all CKD stage groups compared to the controls, even after adjusting for multiple clinical variables. The risk of colorectal and thyroid malignancies was non-linearly associated with CKD stage. Specifically, individuals with “hyperfiltrative” (stage 1) or “advanced” (stage 4/5 without RRT) CKD demonstrated decreased risk of colorectal and thyroid malignancies. A similar non-linear association was also identified for several other cancer types, including liver, lung, and prostate cancers, with CKD stage 1 or 4/5 without RRT associating with a relatively lower risk of cancer. A non-linear association between renal function and adverse clinical outcomes has been reported in other studies but has not been reported previously for malignancy outcomes [24,25]. Both renal hyperfiltration and profound kidney dysfunction have been related to critically increased risk of mortality or cardiovascular events [24,25]. Additionally, the inverse relationship between the risk of cardiovascular and non-cardiovascular outcomes might exist in people with CKD [26–28]. The findings in this study could have been influenced by the creatinine-based calculation of eGFR, as a higher eGFR may be a result of low creatinine, which can be caused by other problems such as low muscle mass. This is partially supported by our results that stage 1 CKD associated with an increased risk of cancer-associated mortality, as cancer cachexia is an important prognostic factor that is independent from body mass index [29]. However, future studies should examine the mechanism of the non-linear association between renal function and risk of cancer.
The presence of baseline CKD was associated with worse prognosis for certain types of subsequently diagnosed cancers. Although we could not review the cause-specific mortalities, this is not surprising given the limited cancer therapy options for individuals with impaired renal function and the increased likelihood of comorbidities [2,15,30]. Therefore, given the increasing number of CKD patients, an appropriate treatment strategy for this patient group would be important [31]. On the other hand, the prognoses for certain hematologic malignancies, including leukemia and multiple myeloma, were better in the CKD population. This may be due to a limitation of our study in that we did not discriminate the subtypes or stages of the studied malignancies. Also, information on cancer treatment was not included. Therefore, chronic leukemia or indolent course myeloma might have been included in these groups considering the older study population, making these results inconclusive. Additionally, this study could inherently contain selection bias, as only those who received multiple general health screenings were included. Individuals with illness and those who were already on follow-up with their attending hospitals would be less likely to receive the nationwide exam. Future studies that involve detailed collection of information, including cancer type, stage, and treatment, are necessary to fully investigate the association between renal dysfunction and cancer prognosis.
Several points need to be interpreted with caution in our study. First, although we reported the cancer incidences in one of the largest cohorts of pre-dialysis CKD, the study is a single-nation study. As cancer epidemiology varies among countries, these results may not be applicable to other countries [21–23]. Second, use of the national health screening program might be affected by the presence of CKD, and patients with serious illness would likely be on follow-up at the attending hospitals and not receive general health screening. Therefore, this could have resulted in selection bias. In addition, the likelihood of cancer screening may be different in CKD patients compared to the general population [32,33], which could have also introduced sample bias. This is particularly possible for malignancies such as thyroid cancer in which screening behavior affects its incidence [34]. Third, results regarding post-malignancy mortality are inconclusive, as detailed subtypes or stages of the cancer types were not available, follow-up duration was limited, and cancer-specific mortality data were not available in our study. Also, it remains unclear whether the defined duration of 3 years adequately reflects actual cancer-associated mortality. Lastly, our study could not provide an explanation for the lower risk of stomach cancer in pre-dialysis CKD patients when compared to the controls or the non-linear association between cancer risk of certain malignancy types and CKD stage. Our inability to provide these mechanisms renders this study descriptive, so further study in another large cohort with additional information to assess mechanism is necessary.
In conclusion, increased risk of hematologic and urinary system malignancies was observed in pre-dialysis CKD. The incidence of digestive and thyroid cancers was lower in individuals with pre-dialysis CKD than in the matched control group and the risk of certain cancer types showed a non-linear association with the stages of CKD. Taken together, healthcare providers should be aware of the diverse risk of various cancers in patients with pre-dialysis CKD.
Supplementary Data
Acknowledgments
The study used the database from the NHIS (No. 2018-1-135). This study was supported by a grant from the Korean Society of Nephrology (GAMBRO 2012).
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.