Physicians’ perceptions of asymptomatic hyperuricemia in patients with chronic kidney disease: A questionnaire survey
Article information
Abstract
Background
Hyperuricemia is associated with the development and progression of chronic kidney disease (CKD) as well as cardiovascular diseases. However, there is no consistent recommendation regarding the treatment of asymptomatic hyperuricemia (AHU) in CKD patients. Here, we surveyed Korean physicians’ perceptions regarding the diagnosis and management of AHU in CKD patients.
Methods
Questionnaires on the management of AHU in CKD patients were emailed to regular members registered with the Korean Society of Nephrology.
Results
A total of 158 members answered the questionnaire. Among the respondents, 49.4%/41.1% were considered hyperuricemic in male CKD patients whereas 36.7%/20.9% were considered hyperuricemic in female CKD patients when defined by serum uric acid level over 7.0/8.0 mg/dL, respectively. A total of 80.4% reported treating AHU in CKD patients. The most important reasons to treat AHU in CKD patients were renal function preservation followed by cerebro-cardiac protection. Majority of respondents (59.5%) thought that uric acid-lowering agents (ULAs) were the most effective method for controlling serum uric acid levels. Approximately 80% chose febuxostat as the preferred medication. A total of 32.3% and 31.0%, respectively, initiated ULA treatment if the serum uric acid level was more than 8.0 or 9.0 mg/dL, respectively. In addition, 39.2% and 30.4% answered that target serum uric acid levels of less than 6.0 or 7.0 mg/dL, respectively, were appropriate. The two major hurdles to prescribing ULAs were concerns of adverse reactions and the existing lack of evidence (i.e., the absence of Korean guidelines).
Conclusion
Most Korean physicians treat AHU in CKD patients to prevent CKD progression and cerebro-cardiovascular complications.
Introduction
Hyperuricemia is a very common biochemical aberration and the prevalence is increasing due to changes in diet, an aging population, and earlier screenings [1]. Uric acid comes from both internal (muscle, liver, intestine) and external (diet) sources, with two-thirds excreted through the kidneys [2]. Approximately 20% to 25% of adult men experience hyperuricemia, and this prevalence increases up to more than 60% in advanced chronic kidney disease (CKD) patients because of their decreased urinary uric acid excretion [1]. Two-thirds of hyperuricemia cases remain asymptomatic. Asymptomatic hyperuricemia (AHU) presents as elevated serum uric acid levels without symptoms or signs of monosodium urate crystal deposition disease, hypertension, CKD, cardiovascular disease, or insulin resistance syndrome.
Although the incidence of hyperuricemia and gout in Korean adults is also increasing, epidemiologic studies remain limited. The prevalence of hyperuricemia (> 7.0 mg/dL) was 4.6% in a hospital medical check-up cohort in 2003 [3]. A cohort of 10,802 hyperuricemia-free men aged 30 to 59 years who underwent annual or biennial medical check-ups in a hospital from 2002 to 2009 revealed an incidence rate of hyperuricemia (> 7.0 mg/dL) of 48.7 per 1,000 person-years (95% confidence interval, 46.8–80.7) [4]. More recently, a report on the prevalence of hyperuricemia using data from the 2016 Korean National Health and Nutrition Examination Survey was released, where the age-standardized prevalence of hyperuricemia in the general Korean population was 11.4% (17.0% in men and 5.9% in women) [5]. Unfortunately, however, there was no evidence of the incidence or prevalence of hyperuricemia in Korean CKD patients.
Hyperuricemia is thought to be associated with decreased renal function through increased oxidative stress, increased endothelial dysfunction, systemic glomerular hypertension, and reduced renal blood flow [6]. Decreased renal function aggravates uric acid excretion impairment in the kidneys and is cyclical in nature. Several studies have reported that hyperuricemia is associated with the development and progression of CKD as well as cardio-metabolic conditions [7–10]. Recently, the Chronic Renal Insufficiency Cohort (CRIC) study revealed that a relationship between baseline serum uric acid levels and progression to end-stage renal disease (ESRD) requiring dialysis and transplantation [11]. Higher baseline serum uric acid levels were associated with more renal outcomes, and the relationship was stronger in patients with an estimated glomerular filtration rate (eGFR) of 45 mL/min/1.73 m2 or more.
We hypothesize that lowering the serum uric acid level could delay renal disease progression and reduce cardiovascular complications. In addition, an effective way to control serum uric acid levels is the prescription of uric acid-lowering agents (ULAs) such as allopurinol, febuxostat, and uricosuric agents.
The Japanese Society of Gout and Nucleic Acid Metabolism recommends treating AHU when either serum uric acid level is greater than 9 mg/dL or when serum uric acid is greater than 8 mg/dL and is accompanied by renal damage, urinary lithiasis, hypertension, ischemic heart disease, diabetes, or metabolic syndrome [12]. However, uric acid-lowering therapy for AHU is not recommended for use in the United States (US) and Europe because of conflicting results [13]. Separately, the Korean Rheumatology Society recommends a combination of guidelines for the management of AHU [14]; specifically, they suggest treating AHU if the serum uric acid level is greater than 9 mg/dL and to search for causes and lifestyle modifications at six months prior to treatment if the serum uric acid level is between 7.0 and 9.0 mg/dL. However, there are no specific Korean guidelines for the management of AHU in CKD patients.
The practice pattern of the management of AHU in CKD patients has not yet been surveyed. This study aimed to clarify how Korean nephrologists manage AHU in CKD patients.
Methods
Study design
We performed a questionnaire survey to determine physicians’ perceptions of AHU in CKD patients from July 9, 2018 to August 6, 2018. We sent and received emails to and from regular members registered with the Korean Society of Nephrology (KSN). The questionnaire was designed as follows: 1) definition of hyperuricemia in CKD patients (> 6.0, > 7.0, > 8.0, > 9.0, and > 10.0 mg/dL); 2) necessities and effective methods of lowering serum uric acid levels (yes vs. no); 3) the purpose of managing AHU (gout prevention, nephrolithiasis prevention, renal preservation, and cerebro-cardiovascular protection); 4) evaluation of the urinary uric acid excretion (yes vs. no); 5) the initial (> 6.0, > 7.0, > 8.0, > 9.0, > 10.0 mg/dL) and the target (< 4.0, < 5.0, < 6.0, < 7.0, < 8.0 mg/dL) serum uric acid levels for prescribing ULAs; 6) preferred medication (allopurinol vs. febuxostat vs. benzbromarone vs. others); 7) evaluation of the HLA-B58:01 allele before prescribing allopurinol (yes vs. no); 8) prescribing doses of allopurinol and febuxostat (open question about the initial and the maximum doses); and 9) hurdles to prescribing ULAs (multiple-choice format [choices of two out of seven], including nonpharmacologic intervention first, too many concomitant medications, fear of adverse reactions, unclear treatment effects, the lack of evidence [i.e., the absence of Korean guidelines], patients’ refusal, not fitting insurance criteria).
This study was approved and got a waiver of informed consent by the Institutional Review Board of the National Medical Center in Seoul, Korea (H-1811-096-001).
Statistical analysis
Descriptive statistics were used to summarize survey responses. The chi-square test was used to compare categorical variables between groups. All statistical analyses were performed with the IBM SPSS Statistics version 20.0 software program (IBM Corp., Armonk, NY, USA). A P value less than 0.05 was considered to be statistically significant.
Results
We sent email communications to a total of 1,258 regular members who were registered with the KSN and received answers back from 158 (12.6%) of them.
The mean (median) age of the study participants was 45 (43) years old, and 100 (63.3%) of the participants were men. Most of the participants were affiliated with referral hospitals (secondary hospitals [34.8%], tertiary or university-associated hospitals [48.7%]). The baseline characteristics of the participants are summarized in Table 1.
Definition of hyperuricemia in CKD patients
Among the respondents, 3.2%, 49.4%, 36.8%, 8.9%, and 1.9% were considered hyperuricemic in male CKD patients to be greater than 6.0, 7.0, 8.0, 9.0, and 10.0 mg/dL, respectively, while 27.2%, 41.1%, 20.9%, 8.0%, and 1.3% were considered hyperuricemic in female CKD patients to be greater than 6.0, 7.0, 8.0, 9.0, and 10.0 mg/dL (Fig. 1). Ninety-three (58.9%) respondents followed the same criteria for hyperuricemia in both male and female CKD patients (> 6.0 mg/dL, five respondents; > 7.0 mg/dL, 44 respondents; > 8.0 mg/dL, 30 respondents; > 9.0 mg/dL, 11 respondents; > 10 mg/dL, three respondents, respectively). Fifty-eight (36.7%) and four (2.5%) respondents chose 1 and 2 mg/dL higher serum uric acid levels in male CKD patients than in female CKD patients, respectively. There was no significant difference in the definition of hyperuricemia among male and female CKD patients according to the hospital hierarchy (linear-by-linear association P = 0.1 and 0.6 for male and female CKD patients, respectively) (Fig. 2).
Intervention for AHU in CKD patients
A majority of respondents (80.4%) reported treating AHU in CKD patients. There was no significant difference in treating AHU according to the hospital hierarchy (88.5 vs. 76.4 vs. 80.5%) (linear-by-linear association P = 0.7).
When we separated the condition according to eGFR, 7.6%, 8.2%, and 18.4% of respondents disagreed about treating AHU in patients with eGFR values of less than 30, 30 to 60, and 60 mL/min/1.73 m2 or more, respectively. ULAs were thought to be the most effective method of controlling serum uric acid levels (59.5%), followed by lifestyle modification (31.0%) and a change in prescription to alternative medicines (9.5%). The most important reasons for treating AHU in CKD patients were renal function preservation followed by cerebro-cardiovascular protection (Table 2).
Evaluation of hyperuricemia
Only 15.2% evaluated urinary uric acid excretion by using 24-hour urine uric acid, fractional excretion of uric acid from random urine, and urinary stone evaluation through abdominal sonography. In addition, only 4.4% performed the HLA-B*58:01 allele test, which is the marker for allopurinol-induced severe cutaneous adverse reactions such as Stevens–Johnson syndrome and toxic epidermal necrolysis.
Prescription of uric acid-lowering agents
Regarding ULA type, 78.1%, 19.7%, and 2.2% of respondents preferred febuxostat, allopurinol, and benzbromarone, respectively. A total of 32.3% and 31.0% of the respondents answered they start the prescription of ULAs when serum uric acid level was greater than 8.0 or 9.0 mg/dL, respectively. Additionally, 39.2% and 30.4% of participants targeted serum uric acid levels of less than 6.0 or 7.0 mg/dL, respectively (Fig. 3; Supplementary Table 1 and 2, available online).
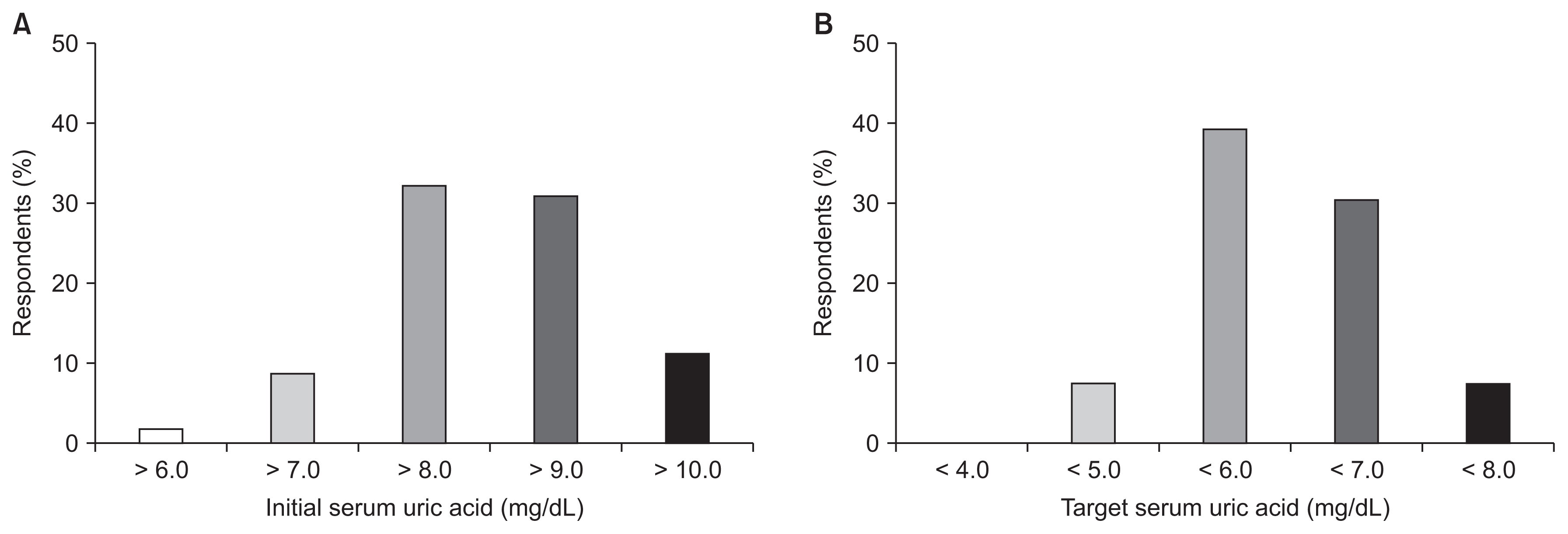
The initial (A) and the target (B) level of serum uric acid in prescribing uric acid-lowering agents.
Hurdles to prescribing ULAs were as follows: 1) use of a nonpharmacologic intervention first (9.7%), 2) too many concomitant medications (0.6%), 3) fear of adverse reactions (36.1%), 4) unclear treatment effects (11.0%), 5) lack of evidence (i.e., the absence of Korean guidelines) (36.5%), 6) refusal by patients (5.5%), and 7) Not fitting insurance criteria (0.6%) (Fig. 4). The initial and maximum dosages of allopurinol (Fig. 5) and febuxostat (Fig. 6) were higher in patients with an eGFR value of 30 mL/min/1.73 m2 or more than in patients with an eGFR of less than 30 mL/min/1.73 m2 (all P values < 0.001).
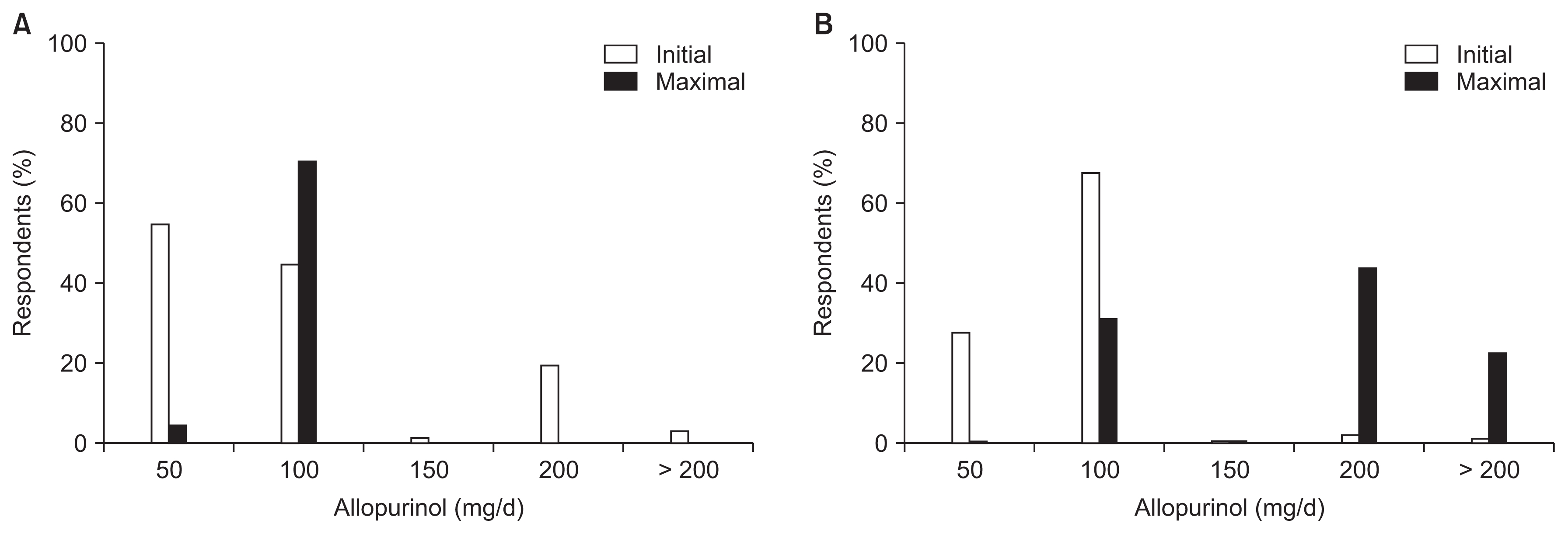
Initial and maximum dosages of allopurinol used to treat asymptomatic hyperuricemia according to renal function.
(A) eGFR < 30 mL/min/1.73 m2; (B) eGFR ≥ 30 mL/min/1.73 m2.
eGFR, estimated glomerular filtration rate.
Discussion
To our knowledge, this study is the first survey concerning the management of AHU in CKD patients in Korea. However, the low response rate to the survey reflects the idea that hyperuricemia in CKD patients is not a priority subject even within the KSN.
About half of the responders considered hyperuricemia in CKD patients to be greater than 7.0 mg/dL of serum uric acid, although approximately one-third chose greater than 8.0 mg/dL of serum uric acid, regardless of sex, and another one-third chose greater than 6.0 mg/dL of serum uric acid in female CKD patients. Most respondents (80.4%) attending CKD patients replied that uric acid-lowering therapy for AHU in CKD patients is necessary. Approximately, 84–89% and 63% of Japanese nephrologist agreed to treat AHU in patients with stages 3 to 5 CKD and stage 5D CKD patients, respectively, while only 4% of rheumatologists in the US prescribed ULAs to patients with AHU [15,16]. The most important reasons for treating AHU in CKD patients included the prevention of CKD progression or cerebro-cardiovascular complications. A very small proportion of respondents evaluated urinary uric acid excretion and the HLA-B*58:01 allele. A majority of participants selected febuxostat as the preferred ULA treatment.
About two-thirds of respondents stated that they prescribed ULAs if the serum uric acid level was greater than 8.0 mg/dL and targeted a serum uric acid level of less than 7.0 mg/dL (< 6.0 mg/dL, 39.2% and < 7.0 mg/dL, 30.4%). Nakaya et al [15] evaluated the initial and target levels of serum uric acid according to CKD stage (CKD stages 3–5 and 5D) and the purpose of treatment (e.g., prevention of gout, urolithiasis, CKD, cardiovascular events [CVEs]). The target levels were different according to every CKD stage and the purpose of treatment. Generally, the initial level of serum uric acid to treat AHU increased as renal function decreased (from 8.1 to 9.5 mg/dL). However, the target level of serum uric acid was approximately 7.0 mg/dL irrespective of CKD stage, except for in the case of CKD 5D (8.2 mg/dL for gout and 7.6 mg/dL for CVE). We did not separate the patients’ conditions according to CKD stage due to the fear of a low response rate related to difficult questions. Further surveillance or cohort studies to deduce the target levels of serum uric acid according to CKD stage and the purpose of treatment are required.
Fear of adverse reactions and the lack of evidence are important hurdles to prescribing ULAs. In addition, the dosage of ULAs was inversely associated with the eGFR.
As mentioned above, several studies have reported an association between hyperuricemia and CKD [7–9]. In addition, there have been many clinical studies on serum uric acid reduction and its effect on renal function. However, most of them were limited by a small size and short follow-up duration. In addition, the results regarding renal function and proteinuria were inconsistent [6]. Therefore, there has been no strong recommendation made for treating AHU, even in CKD patients. However, there have been many clinical studies published after the official recommendations from Japan, the US, and Europe suggesting an effect of allopurinol on endothelial dysfunction improvement, GFR preservation, improvement of insulin resistance and systemic inflammation, and increased patient and graft survival in kidney recipients in patients with AHU [17–20].
In addition, several trials on the effects of febuxostat, topiroxostat, and newer ULAs on renal function and cardiovascular diseases have been published. Both febuxostat and topiroxostat delayed eGFR decline [21,22]. In addition, two recently released large clinical trials (the Febuxostat for Cerebral and Cardiorenovascular Events Prevention Study [FREED] and the Febuxostat versus Placebo Randomized Controlled Trial Regarding Reduced Renal Function in Patients with Hyperuricemia Complicated by Chronic Kidney Disease Stage 3 [FEATHER]) [23,24] using febuxostat suggested a target serum uric acid level, which is beneficial in lowering mortality and renal progression and defined CKD patient groups who can benefit from ULAs. Febuxostat effectively reduced serum uric acid levels in both trials in 12 weeks and maintained decreased serum uric acid levels until the end of the respective investigations. Both composite cerebro-CVEs and renal outcomes occurred less frequently in the febuxostat group in the FREED trial, and the study verified successful reduced serum uric acid level outcomes (serum uric acid between 5.0 and 6.0 mg/dL) [23]. Patients with mild kidney injuries, i.e., those without proteinuria and serum creatinine levels less than the median level of all study participants, showed slower eGFR progression in the FEATHER trial [24].
Nephrologists manage CKD patients by using renin angiotensin aldosterone receptor blockers, statins, and other verified renoprotective medicines, but many CKD patients still progress to ESRD. Thus, another option to improve renal and patient outcomes in CKD patients is needed. Our focus has therefore expanded to uremic toxins and inflammatory mediators. Moreover, the management of serum uric acid is an attractive option based on experimental and clinical evidence. Currently, the CKD FIX (a placebo-controlled study giving allopurinol to CKD patients in stages 3 and 4 with proteinuria) [25] and PERL (a placebo-controlled study giving allopurinol to type 1 diabetic nephropathy patients with eGFRs of 40–100 mL/min/1.73 m2) [26] trials are actively ongoing, and we expect to confirm from these more clear evidence regarding the relationship between lowering serum uric acid level and renal and patient outcomes for CKD patients with a variety of conditions.
Although decreased renal function can increase the serum uric acid level and a cohort or a prospective study to find the causal relationship between AHU and patient outcomes in Korean CKD patients is needed, we think that the systemic impact of hyperuricemia does not differ with the presence of CKD. Therefore, we carefully suggest adopting the definition of hyperuricemia as a serum uric acid level of greater than 7.0 mg/dL for the general population, irrespective of CKD stage. In addition, we suggest prescribing ULAs if the serum uric acid level exceeds 8.0 mg/dL with the consideration of tissue solubility of uric acid and targeting the serum uric acid level to less than 6.0 mg/dL, as is the guideline for gout patients. However, more research is needed to establish a conclusion regarding the management of AHU in CKD patients.
This survey has a limitation, in that only 12.6% of members responded to the questionnaire. Therefore, the findings of this report may not represent the real practice patterns of nephrologists who care for CKD patients in Korea.
In conclusion, a number of registered members in the KSN caring for CKD patients treat AHU to prevent CKD progression or cerebro-cardiovascular complications mainly by using febuxostat and allopurinol. We must establish and share Korean guidelines for the management of AHU in CKD patients.
Supplementary Information
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Ran-hui Cha, Chul Woo Yang, and Duk-Hee Kang participated in conception or design. Ran-hui Cha participated in analysis and interpretation of data, and drafting the article or revising. Su Hyun Kim, Eun Hui Bae, Mina Yu, Beom Soon Choi, Hoon Young Choi, Sun Woo Kang, Jungho Shin, and Sang Youb Han participated in providing intellectual content of critical importance to the work. All authors read and approved of the final version to be published.





