Maternal exposure to airborne particulate matter during pregnancy and lactation induces kidney injury in rat dams and their male offspring: the role of vitamin D in pregnancy and beyond
Article information
Abstract
Background
Little is known about the transgenerational effects of maternal exposure to fine particulate matter (PM2.5) on offspring kidney health. This study investigated the effect of maternal administration of PM2.5 or PM2.5 with vitamin D during pregnancy and lactation on renal injury in rat dams and their offspring.
Methods
Nine pregnant Sprague-Dawley rats received oral administration of normal saline, airborne PM2.5, or PM2.5 with vitamin D from gestational day 11 to postpartum day 21. Kidneys of rat dams (n = 3 for each group) and their male offspring (n = 5 for each group) were taken for analysis on postpartum or postnatal day 21.
Results
Maternal PM2.5 exposure increased glomerular damage, tubulointerstitial injury, and cortical macrophage infiltration in both dams and pups; all increases were attenuated by vitamin D administration. In dam kidneys, PM2.5 increased the protein expression of vitamin D receptor (VDR), klotho, and tumor necrosis factor-α; vitamin D lessened these changes. The expressions of renin, nuclear factor erythroid 2-related factor 2 (Nrf2), and nuclear factor-kappa B (NF-κB) p50 decreased in rat dams exposed to PM2.5. In offspring kidneys, exposure to maternal PM2.5 reduced the expression of VDR, renin, angiotensin-converting enzyme (ACE), Nrf2, and NF-κB p50, but increased cytochrome P450 24A1 expression. Maternal vitamin D administration with PM2.5 enhanced VDR, ACE, and NF-κB p50 activities in pup kidneys.
Conclusion
PM2.5 exposure during nephrogenesis may exert transgenerational renal impairment, and maternal vitamin D intake could attenuate PM2.5-induced kidney damage in mothers and their offspring.
Introduction
Ambient particulate matter (PM) poses substantial health burdens worldwide [1]. Exposure to fine PM (PM2.5, particles with aerodynamic diameter of ≤2.5 μm) is associated with increased risk of incident chronic kidney disease (CKD), decreased glomerular filtration rate, and end-stage renal disease [2,3]. There is also increasing evidence showing that in-utero exposure to air pollutants leads to adverse pregnancy outcomes [4]. The detrimental effects of maternal exposure to PM during pregnancy involve placental translocation of fine particles, systemic and placental oxidative stress and inflammation, epigenetic alterations, and endocrine disruption, all of which impact offspring disease later in life [5]. Therefore, perinatal exposure to PM during critical windows of nephrogenesis may cause functional and structural changes in the developing kidney and prime the kidney for subsequent CKD [6].
Airborne PM facilitates the development of renal injury through renin-angiotensin system (RAS) imbalance, and various molecular mechanisms are associated with oxidative stress, inflammation, DNA damage, etc. [7]. In mice exposed to PM2.5, the activities of angiotensin-converting enzyme (ACE) and angiotensin II type 1 receptor (AT1R) have been found to be increased in renal tissue, with such increases preceding oxidative damage and inflammatory responses in the kidney [8]. Notably, maternal exposure to PM2.5 during pregnancy has been shown to reduce placental angiotensin II as well as placental mass and size, indicating that altered placental RAS following maternal PM exposure may impair fetal growth [9]. The RAS is essential for maintaining normal gestation, and circulating RAS components are elevated in an uncomplicated pregnancy [10]. Intrarenal renin activity in a newborn is ~20 times higher than that in an adult [11], and activation of the intrarenal RAS is necessary for normal kidney development [12]. Meanwhile, there are significant changes in vitamin D metabolism during pregnancy, and maternal serum levels of active vitamin D exhibit increases beginning early in pregnancy [13]. Vitamin D is involved in several pleiotropic effects, including the regulation of cell proliferation and differentiation, anti-inflammation, and renin gene regulation, which are all important for kidney development [14,15]. Ambient PM2.5 is known to disrupt vitamin D homeostasis, and pregnant women exposed to higher levels of PM2.5 are reported to have a lower level of maternal serum 25(OH)D [16].
Little is known about the transgenerational effects of maternal exposure to PM on offspring kidney health, or about the effects of maternal vitamin D administration on PM-provoked renal impairment during nephrogenesis. While kidney development in humans is completed in utero by gestational week 36, nephrogenesis in the rat starts on embryonic day 12 and is completed at ~20 days after birth [17]. The average rat gestation time is 21 to 23 days, and weaning generally occurs about 21 days after birth [18]. Thus, rat kidney development begins mid-gestation and persists throughout lactation. With this background, the present study explored whether maternal PM exposure during rat nephrogenesis can induce kidney injury in rat dams and their offspring, as well as how maternal vitamin D supplementation affects renal impairment provoked by PM exposure.
Methods
Ethical approval
Our animal experiment was approved by the Institutional Animal Care and Use Committee of Korea University (No. KOREA-2021-0047). The experimental protocol was carried out according to the guidelines of the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The authors made all efforts to minimize the animals’ pain and suffering.
Particulate matter collection, analysis, and preparation
Airborne fine PM samples were collected as described in our previous experiment [19]. Two filters (QR-100; Sibata) on the same day (December 22, 2019) above the World Health Organization guidelines for PM2.5 (average of ≥25 μg/m3 per day) were chosen for the experiment. The extraction of PM2.5 samples was filtered with a 0.2-μm syringe filter to remove larger interfering particles prior to the experiment. To prepare for the animal experiment, the PM exposure dose was determined using the following equation, which was adopted from the average daily dose (ADD) calculation of the U.S. Environmental Protection Agency [20];
ADD (mg/kg⋅day) = (Cair × InhR × ET × EF × ED) / (BW × AT)
where Cair, concentration of contaminant in the air (mg/m3); InhR, inhalation rate (m3/day); ET, exposure time (hr/day); EF, exposure frequency (day/yr); ED, exposure duration (yr); BW, body weight (kg); and AT, average time (day).
For maternal PM exposure, 70 μL of PM suspension was prepared for rat dams (one instillation [70 μL] per day × total 21 times = 1.47 mL). The tidal volume and respiration rate in an average person (60 kg) are 500 mL and 14 breaths/min, while the respiration volume for 23 hours is 9,660 L/day (https://www.epa.gov/expobox/exposure-assessment-tools-routes). The suction volume (inhalation flow) of an air sampler is 1,380,000 L/day (1,000 L/min × 23 hours). Since the air sampler collects airborne particles over a period of 23 hours with a 1-hour pause, we calculated the volume for 23 hours. Hence, the airflow mass extracted from the air sampler over 2 days corresponds to a person’s respiration volume over 286 days (1,380,000 L/day × 2 days / 9,660 L/day = 286 days). As two filters collected for a day were extracted into 50 mL of a saline buffer, it was assumed that 0.175 mL of PM suspension represents the amount of daily human exposure (50 mL/286 days = 0.175 mL/day). The total amount given to each rat dam (1.47 mL) was expected to be the same as the volume of human PM exposure in 8.4 days (1.47 mL/0.175 mL/day = 8.4 days).
Animal preparation
Nine pregnant Sprague-Dawley rats on the 10th day of pregnancy (Raonbio Co., Ltd) were kept in standard housing conditions. The pregnant rats were divided into three groups: control, PM2.5, and PM2.5 with vitamin D groups (n = 3, respectively). Rat dams were allowed to deliver their pups naturally on days 21 to 22 of pregnancy. Based on the group to which they had been assigned, the rat dams received saline (70 μL, 5 times/wk), PM2.5 (70 μL dissolved in saline, 5 times/wk), and PM2.5 (70 μL dissolved in saline, 5 times/wk) with vitamin D (cholecalciferol, 1,000 IU/kg, 3 times/wk; FND Net Co.) via an orogastric tube from gestation day 11 to lactation day 21. The pups were maintained without any intervention and weaned at postnatal day 21. Dams and male pups (n = 5, respectively) were sacrificed on postpartum or postnatal day 21. We only used male pups because animal models show that male offspring are more sensitive to fetal insult during development [21]. Maternal urine samples were collected using the metabolic cage 1 day before sacrifice. Dams and pups were anesthetized with 2% isoflurane, and blood was collected through cardiac puncture. The kidneys were then harvested and processed.
Histological examination
Fixed kidneys were embedded in paraffin and sectioned to a thickness of 4 µm. The kidney structures of the dams and pups were examined using hematoxylin and eosin staining. The degree of glomerular damage was scored from 0 to 4 as described by Raij et al. [22] (0, no lesion; 1, <25% of glomerulus damaged; 2, 25% to 50%; 3, 50% to 75%; and 4, >75%). Glomerular damage was determined in 10 randomly selected fields per kidney from each of three (dams) or five (pups) rats at ×200 magnification using a double-blind method. The severity of tubulointerstitial damage was also evaluated in 10 randomly chosen nonoverlapping fields of the cortex per kidney section (×200 magnification) from each of three (for dams) or five (for pups) rats. The tubulointerstitial injury (tubular dilation, atrophy, or cast formation, tubular cell flattening or vacuolization) was scored as follows: 0, no lesion; 1, <25% of tubules injured; 2, 25% to 50% of tubules injured; 3, 50% to 75% of tubules injured; and 4, >75% of tubules injured. All of the stained slides were digitalized using Pannoramic Scan II (3DHISTECH; Sysmex) and quantified on digitalized images (CaseViewer; 3DHISTECH, ver. 2.2.0).
Western blotting
Renal tissue was lysed in the T-PER Tissue Protein Extraction Reagent (Thermo Scientific) and the Xpert Protease Inhibitor Cocktail Solution (100×; GenDEPOT) using a homogenizer. Bicinchoninic acid protein assay was used to determine the protein concentration as follows: 20 µg of protein was separated on 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, then transferred onto polyvinylidene fluoride membranes. Next, the membranes were incubated overnight at 4 °C with primary antibodies against vitamin D receptor (VDR) (Abcam), klotho (Invitrogen), cytochrome P450 mixed-function oxidase (CYP) 27B1 (Abcam), CYP24A1 (Invitrogen), renin (Santa Cruz Biotechnology), ACE (Invitrogen), AT1R (Invitrogen), nuclear factor erythroid 2-related factor 2 (Nrf2) (Abcam), nuclear factor-kappa B (NF-κB) p50 (Santa Cruz Biotechnology), and tumor necrosis factor alpha (TNF-α) (Invitrogen). The blots were then incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G (Cell Signaling Technology) at room temperature for 2 hours. The ChemiDoc Touch Imaging System (Bio-Rad Laboratories) was used for imaging of the western blots. The intensity of the identified lanes was quantified using densitometry. The levels of each protein were expressed relative to GAPDH.
Immunohistochemistry
Three kidneys in each group were selected to obtain representative immunohistochemistry (IHC) of VDR, klotho, CYP24A1, renin, ACE, Nrf2, NF-κB p50, and TNF-α. Antigen retrieval was achieved with a Tris-ethylenediaminetetraacetic acid pH 8.0 buffer, and most slides were stained with a Dako AutoStainer (Dako). Primary antibodies against VDR (Santa Cruz Biotechnology), klotho (Invitrogen), CYP24A1 (Invitrogen), renin (Santa Cruz Biotechnology), ACE (Invitrogen), Nrf2 (Novus), NF-κB p50 (Santa Cruz Biotechnology), and TNF-α (Invitrogen) were used. These antibodies were incubated for 40 minutes at room temperature and washed three times in tris-buffered saline for 5 minutes. The Novolink polymer detection system (RE7150-K) was employed according to the manufacturer’s instructions. To analyze the infiltrations of monocytes/macrophages, IHC against the rat monocyte-specific marker CD68 (Invitrogen) was also performed. CD68-positive cells were counted in 10 random nonoverlapping fields of cortex per section from each of three or five rats.
Enzyme-linked immunosorbent assay
The concentrations of 25(OH)D, Ca2+, and neutrophil gelatinase-associated lipocalin (NGAL) in blood along with the concentrations of NGAL, albumin, and creatinine in urine were measured using commercial enzyme-linked immunosorbent assay (ELISA) kits; 25(OH)D ELISA kit (MyBioSource), calcium assay kit (Abcam), rat NGAL ELISA kit (BioPorto), rat albumin ELISA kit (MyBioSource), and creatinine assay kit (R&D systems), respectively. The urine levels of NGAL and albumin were adjusted for urine creatinine.
Statistical analysis
All experiments were conducted at least three times. The results were presented as means ± SEM. Statistical analysis was performed with GraphPad Prism ver. 7.0. All data were analyzed using the one-way analysis of variance, Tukey multiple comparison test, or Student t test. A p-value of <0.05 was considered statistically significant.
Results
Characteristics of particulate matter
The PM sample used in this study was collected on the day with an ambient PM2.5 concentration of 38 μg/m3. The levels of water-soluble organic carbon and inorganic ions in the PM suspension were similar to those described in our previous report [19]. NO3− was the most abundant ion, followed by NH4+, organic carbon, Na+, SO42–, etc. (Table 1).
Body weight changes and laboratory data
Rat dams and pups showed similar body weights among groups on postpartum or postnatal day 21. Serum 25(OH)D levels were also similar between groups. However, exposure to maternal PM2.5 reduced the serum calcium levels of pups compared to those of the controls. Maternal calcium concentration was higher in the PM2.5 group compared to the PM2.5 with vitamin D group. The levels of NGAL and albumin to creatinine in urine were not different among the groups (Table 2).
Renal histological alterations
Maternal PM2.5 exposure induced glomerular and tubular abnormalities in the kidneys of both dams (Fig. 1A–E) and pups (Fig. 1F–J). Histologic analysis exhibited higher scores of glomerular damage and tubulointerstitial injury in the PM2.5-treated dams and pups (Fig. 1D, E, I, J; p < 0.05 for all). Glomerular mesangial expansion, tubular cast formation or degeneration, and interstitial alterations could all be seen in rat kidneys following maternal PM2.5 exposure (Fig. 1B, G). These changes were ameliorated by maternal vitamin D treatment in both dams and pups (Fig. 1C, H).

Renal histological alterations in rat dams (A–E) and their male pups (F–J).
Dams and pups exposed to PM2.5 showed prominent renal structural changes (B, dams; G, pups) (arrows, sclerotic glomeruli; arrowheads, degenerated or dilated tubules) when compared to corresponding control kidneys (A, dams; F, pups). Maternal vitamin D (Vit D) supplementation ameliorated renal injury in both dams (C) and pups (H), as evidenced by the scores of glomerular damage (D, dams; I, pups) and tubulointerstitial injury (E, dams; J, pups). (A–C and F–H) Hematoxylin and eosin stain, ×100; bar = 100 µm (n = 3–5 for each group).
PM, particulate matter.
*p < 0.05, control vs. PM2.5 or PM2.5 + Vit D; #p < 0.05, PM2.5 vs. PM2.5 + Vit D.
Vitamin D receptor, klotho, CYP27B1, and CYP24A1 expression
To investigate the changes in vitamin D signaling by maternal PM2.5 exposure, we measured intrarenal VDR, klotho, CYP27B1, and CYP24A1 expressions in dams and pups at the weaning (Fig. 2, 3). Exposure to PM2.5 increased the protein expression of VDR and klotho in dam kidneys, and these upregulations were reduced by vitamin D treatment (Fig. 2A, E). VDR (Fig. 2B–D) and klotho (Fig. 2F–H) expressions were strong in the tubular cells and some glomeruli of PM2.5-exposed rat kidney, and these elevated expressions were reduced by vitamin D treatment. CYP27B1 (Fig. 2I) and CYP24A1 (Fig. 2J) expressions were not different among the dam groups. By contrast, VDR expression was noticeably suppressed in the kidneys of pups exposed to maternal PM2.5; this suppressed expression was restored by vitamin D treatment (Fig. 3A–D). Intrarenal CYP24A1 expression was upregulated in the pups of the PM2.5 group, and this change was not reduced by maternal vitamin D intake (Fig. 3E–H). There were no differences in klotho (Fig. 3I) or CYP27B1 (Fig. 3J) activities in pup kidneys.

Intrarenal vitamin D (Vit D) signaling in rat dams.
(A–D) Vitamin D receptor (VDR), (E–H) klotho, (I) CYP27B1, and (J) CYP24A1. Western blot analysis revealed increases in VDR (A) and klotho (E) activities in PM2.5-exposed dam kidneys as compared to the controls, which were reversed by Vit D treatment. Compared to the control rats (B), VDR was more strongly expressed in the dilated tubular epithelial cells and macula densa of the juxtaglomerular apparatus in PM2.5-exposed dam kidneys (C; arrows). Klotho was also highly expressed in almost all major tubular segments and glomeruli of PM2.5-exposed kidneys (F, controls; G, PM2.5-exposed kidneys, arrows). Maternal Vit D supplementation lessened both VDR (D) and klotho (H) expressions in the renal cortex. (B–D and F–H) Immunohistochemistry; ×200, bar = 50 µm (n = 3 for each group). No difference was observed in CYP27B1 (I) and CYP24A1 (J) expressions among the groups (*p < 0.05, control vs. PM2.5; #p < 0.05, PM2.5 vs. PM2.5 + Vit D).
CYP, cytochrome P450 mixed-function oxidase; PM, particulate matter.
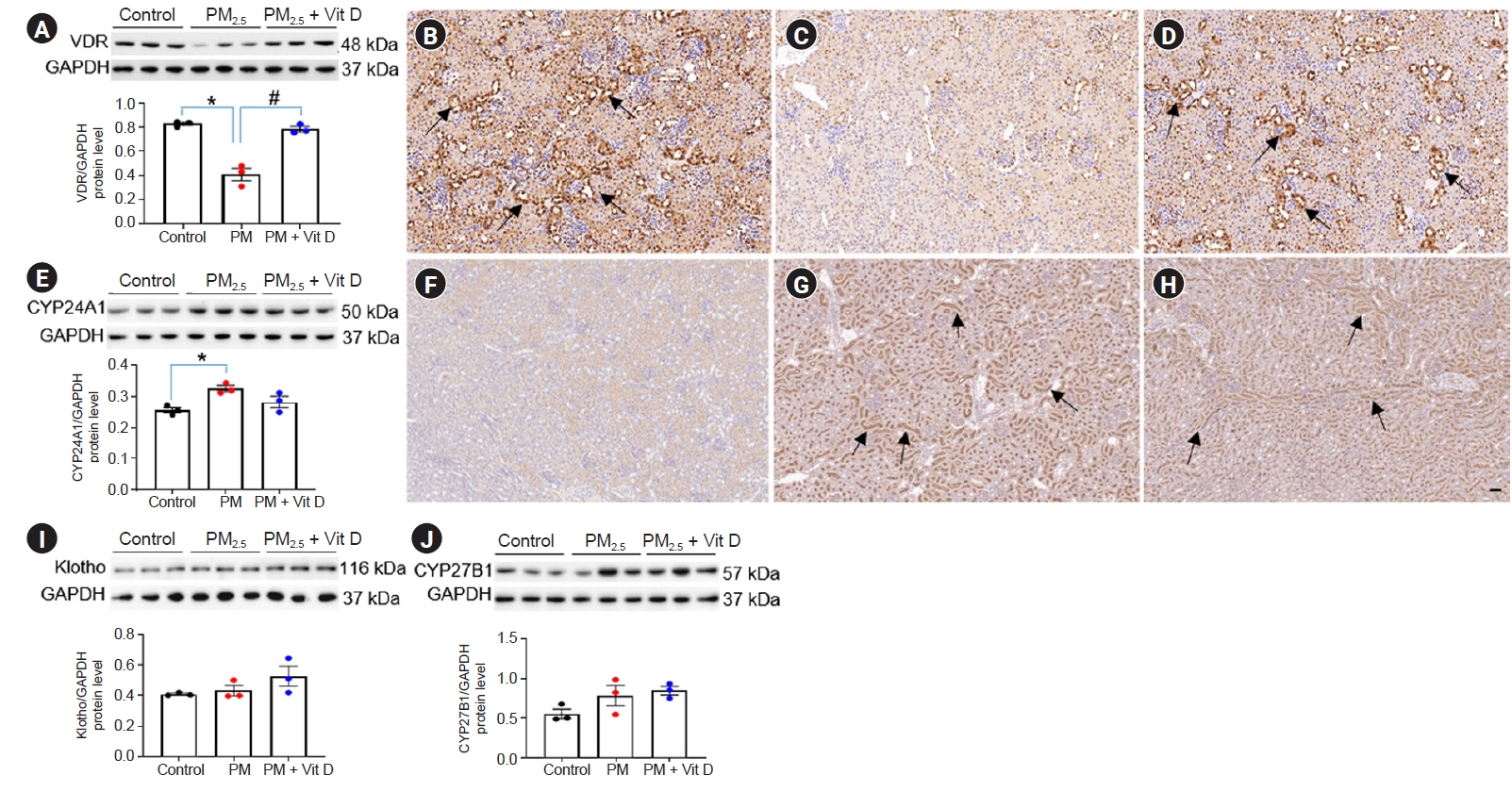
Intrarenal vitamin D (Vit D) signaling in rat pups.
(A–D) Vitamin D receptor (VDR), (E–H) CYP24A1, (I) klotho, and (J) CYP27B1. Western blot analysis revealed a decrease in VDR (A) and an increase in CYP24A1 (E) in pups from PM2.5-exposed dams as compared to the controls. VDR activity was restored by maternal Vit D supplementation. VDR expression was mainly observed in tubular epithelial cells of control pup kidneys (B, arrows), while it was markedly diminished in kidneys from pups exposed to maternal PM2.5 (C). Maternal Vit D intake restored intrarenal VDR expression in pups at the weaning (D, arrows). While CYP24A1 activity was rarely detected in control pup kidneys (F), it was highly expressed in the brush border of the tubules in pup kidneys exposed to maternal PM2.5 (G, arrows). Maternal Vit D intake did not reduce CYP24A1 expression (H, arrows). (B–D and F–H) Immunohistochemistry; ×200, bar = 50 µm (n = 3 for each group). No difference was observed in the intrarenal activities of klotho (I) and CYP27B1 (J) among the groups (*p < 0.05, control vs. PM2.5; #p < 0.05, PM2.5 vs. PM2.5 + Vit D).
CYP, cytochrome P450 mixed-function oxidase; PM, particulate matter.
Renin, ACE, and AT1R expression
Next, we further investigated alterations of intrarenal RAS signaling after maternal PM2.5 or PM2.5 with vitamin D treatment. Intrarenal renin levels were suppressed in both dams (Fig. 4A–D) and pups (Fig. 5A–D) after maternal PM2.5 exposure. While ACE (Fig. 4E) and AT1R (Fig. 4F) expression did not differ among dam groups, ACE activity (Fig. 5E–H) was reduced in the kidneys of pups in the PM2.5 group. Maternal vitamin D treatment with PM2.5 restored the intrarenal ACE activity in pups. There were no differences in AT1R expression among the pup groups (Fig. 5I).

Intrarenal renin-angiotensin system signaling in rat dams.
(A–D) Renin, (E) angiotensin-converting enzyme (ACE), and (F) angiotensin II type 1 receptor (AT1R). Western blot analysis revealed a decrease in renin activity in PM2.5-exposed dam kidneys as compared to the controls (A). Immunohistochemically, renin expression was found in juxtaglomerular and interstitial cells in control kidneys (B, arrows). PM2.5 exposure reduced intrarenal renin expression (C), and vitamin D (Vit D) supplementation did not restore renin activity in the kidney (A and D). (B–D) Immunohistochemistry; ×200, bar = 50 µm (n = 3 for each group). No difference was observed in the intrarenal activities of ACE (E) and AT1R (F) among the groups (*p < 0.05, control vs. PM2.5 or PM2.5 + Vit D).
PM, particulate matter.

Intrarenal renin-angiotensin system signaling in rat pups.
(A–D) Renin, (E–H) angiotensin-converting enzyme (ACE), and (I) angiotensin II type 1 receptor (AT1R). Western blot analysis revealed decreases in renin (A) and ACE (E) activities in pup kidneys exposed to maternal PM2.5 as compared to the controls. Intrarenal ACE activity was restored by maternal vitamin D (Vit D) supplementation (E). Renin expression was found in juxtaglomerular and interstitial cells in control kidneys (B, arrows) whereas it was rarely detected in pup kidneys of maternal PM2.5 exposure (C) and those of maternal Vit D intake with PM2.5 exposure (D). ACE was also widely expressed throughout the brush border of the proximal tubules in control pup kidneys (F, arrows). Maternal PM2.5 exposure remarkably reduced intrarenal ACE expression (G), while Vit D intake by dams restored ACE expression in the pup kidney (H, arrows). (B–D and F–H) Immunohistochemistry; ×200, bar = 50 µm (n = 3 for each group).
No difference was observed in the intrarenal activity of AT1R among the groups (*p < 0.05, control vs. PM2.5; **p < 0.05, control vs. PM2.5 + Vit D; #p < 0.05, PM2.5 vs. PM2.5 + Vit D).
PM, particulate matter.
Nrf2, NF-κB p50, and TNF-α expression
We next examined the effect of maternal PM2.5 or PM2.5 with vitamin D on renal inflammation and oxidative damage. Maternal exposure to PM2.5 reduced Nrf2 and NF-κB p50 activities in both dam (Fig. 6A–H) and pup (Fig. 7A–H) kidneys while increasing TNF-α expression in dam kidneys (Fig. 6I–L). Nrf2 and NF-κB p50 expressions were high mainly within the tubular cells of control kidneys whereas they were weakly detected in the PM2.5-exposed group. Maternal vitamin D intake with PM2.5 exposure had no obvious effect on Nrf2 expression in kidneys of both dams (Fig. 6B–D) and pups (Fig. 7B–D) whereas it restored NF-κB p50 expression in pup kidneys (Fig. 7E–H). In dam kidneys, PM2.5 administration increased intrarenal TNF-α expression, and this increased expression was abolished by vitamin D intake (Fig. 6I–L). Maternal PM2.5 exposure did not affect TNF-α expression in pup kidneys (Fig. 7I).

Renal oxidative stress and inflammation in rat dams.
(A–D) Nuclear factor erythroid 2-related factor 2 (Nrf2), (E–H) nuclear factor-kappa B (NF-κB) p50, and (I–L) tumor necrosis factor alpha (TNF-α). Western blot analysis revealed decreases in Nrf2 (A) and NF-κB p50 (E) and an increase in TNF-α (I) in PM2.5-exposed dam kidneys as compared to the controls. Vitamin D (Vit D) supplementation reduced intrarenal TNF-α activity (I). While Nrf2 and NF-κB p50 expressions were well observed in control dam kidneys (B and F; arrows, respectively), they were weakly detected in PM2.5-exposed kidneys (C and G, respectively). Vit D intake did not restore their expressions (D and H). Intrarenal TNF-α expression was clearly detected throughout all tubular segments and glomeruli in the PM2.5 group (K, arrows) as compared to the control group (J) and the PM2.5 with Vit D group (L). (B–D, F–H, and J–L) Immunohistochemistry; ×200, bar = 50 µm (n = 3 for each group).
*p < 0.05, control vs. PM2.5; **p < 0.05, control vs. PM2.5 + Vit D; #p < 0.05, PM2.5 vs. PM2.5 + Vit D.
PM, particulate matter.

Renal oxidative stress and inflammation in rat pups.
(A–D) Nuclear factor erythroid 2-related factor 2 (Nrf2), (E–H) nuclear factor-kappa B (NF-κB) p50, (I) tumor necrosis factor alpha (TNF-α). Western blot analysis revealed decreases in Nrf2 (A) and NF-κB p50 (E) activities in pups from PM2.5-exposed dams as compared to the controls. Maternal vitamin D (Vit D) supplementation restored intrarenal NF-κB p50 activity in pup kidneys (E). While the Nrf2 and NF-κB p50 expressions were well observed in control pup kidneys (B and F; arrows, respectively), they were weakly detected in pup kidneys of maternal PM2.5 exposure (C and G, respectively). Maternal Vit D intake did not restore Nrf2 expression in pups (D) whereas it increased the intrarenal expression of NF-κB p50 (H, arrows). (B–D and F–H) Immunohistochemistry; ×200, bar = 50 µm (n = 5 for each group). No difference was observed in the intrarenal activity of TNF-α (I) among the groups (*p < 0.05, control vs. PM2.5; #p < 0.05, PM2.5 vs. PM2.5 + Vit D).
PM, particulate matter.
CD68 expression
CD68 has been widely used as a marker of inflammation associated with cells of macrophage lineage [23]. We evaluated the presence of CD68-positive cells after exposure to maternal PM2.5 or PM2.5 with vitamin D. Maternal PM2.5 exposure increased CD68-positive cell numbers in both dam (Fig. 8A–D) and pup (Fig. 8E–H) kidneys. The CD68-positive macrophages were mainly expressed cytoplasmic on tubulointerstitium, glomerular mesangium, and injured vessel area (Fig. 8B, F). Maternal vitamin D intake following PM2.5 exposure reduced the numbers of infiltrated macrophages in both dams and pups compared to PM2.5-exposed kidneys (Fig. 8D, H).

Renal macrophage infiltration in dams (A–D) and pups (E–H).
Rat dams and their pups exposed to PM2.5 showed prominent cortical macrophage infiltration mainly in tubulointerstitium (B, dams; F, pups; arrows, respectively) as compared to the corresponding control rat kidneys (A, dams; E, pups). Vitamin D (Vit D) supplementation in both dams (C and D) and pups (G and H). (A–C and E–G) Immunohistochemistry; ×400, bar = 20 µm (n = 3–5 for each group).
PM, particulate matter.
*p < 0.05, control vs. PM2.5; #p < 0.05, PM2.5 vs. PM2.5 + Vit D.
Discussion
The major finding of the present study is that maternal exposure to PM2.5 during nephrogenesis induces renal injury in both dams and pups, which may be relevant to disturbances of vitamin D signaling and the RAS. Maternal vitamin D intake attenuates PM2.5-induced kidney damage in dams and pups at weaning. Glomerular and tubular injury, oxidative stress, and inflammation all increased in the kidneys from both dams and pups following maternal PM2.5 exposure, with these increases being lessened by maternal vitamin D intake.
Both RAS activation and vitamin D signaling are important for physiological adaptation during normal pregnancies [10,14] and their alterations lead to impaired renal development [15]. Pups from dams fed to a vitamin D-deficient diet during nephrogenesis show renal disturbances in development associated with RAS alterations [24]. Pregnant women exposed to higher levels of air pollution showed greater correlations of vitamin D deficiency [16]. Studies have also revealed that exposure to PM2.5 alters placental RAS and impairs fetal health [9,25]. Therefore, we evaluated renal risks of maternal PM2.5 exposure in association with vitamin D signaling and RAS activity in both dams and pups. While maternal PM2.5 exposure did not affect serum levels of 25(OH)D and renal function in dams, there were significant glomerular and tubulointerstitial changes in PM2.5-exposed dam and offspring kidneys. These injuries were alleviated by maternal vitamin D intake in both dams and pups.
Vitamin D [25(OH)D] is crucial for mineral homeostasis, having the capacity to regulate diverse physiological activities [26]. VDR is a transcription factor mediating most known biological actions of vitamin D [26]. VDR in the renal proximal tubule cells regulates 1,25(OH)2D3 production [27], and decreasing VDR activation in the kidney is found in hypocalcemic conditions [28]. Biologically active vitamin D [1,25(OH)2D3] is mostly synthesized in the mitochondria of proximal tubular cells by 1α-hydroxylase (CYP27B1), while the hormone activities are mainly controlled by its catabolizing enzyme 1,25(OH)2D-24-hydroxylase (CYP24A1) [26]. Several factors, including fibroblast growth factor 23, tightly regulate renal vitamin D metabolism [29]. As both an anti-aging protein and the coreceptor for fibroblast growth factor 23, klotho has been shown to protect the kidney by inhibiting the RAS in diseased kidneys [30]. In this study, we found opposing results for intrarenal VDR activity in dams and offspring after maternal PM2.5 exposure; i.e., we found increasing activity in dams and decreasing expression in pups. Dams exposed to PM2.5 also showed increased expression of klotho in tubular cells, and vitamin D supplementation reversed renal activities of VDR and klotho genes in dams. In pups, maternal PM2.5 exposure increased catabolizing enzyme CYP24A1 activity and decreased serum calcium level. Intriguingly, intrarenal renin suppression occurred in both dams and pups, while subsequent ACE loss was found in the pups of PM2.5-treated dams. Maternal vitamin D intake restored intrarenal VDR and ACE activity in the offspring, suggesting its potential role in RAS regulation during renal development. Our previous in vitro study [19] demonstrated that PM2.5 exposure reduced VDR expression in human proximal renal tubular cells and increased the activities of CYP27B1, renin, ACE, and AT1R. Vitamin D analog reversed the RAS activation, while it restored VDR expression and reduced CYP27B1 activity. In this study, unexpectedly, intrarenal VDR and klotho were activated whereas intrarenal renin was repressed by PM2.5 exposure in dams. Dams could show renal compensation for PM2.5 exposure during the nephrogenic period, given that VDR and klotho protect against kidney injury through various mechanisms, including anti-inflammation, inhibition of renal fibrogenesis, and RAS suppression [30–32]. By contrast, maternal exposure to PM2.5-induced intrarenal VDR loss in pups following the completion of nephrogenesis. VDR activation for protection against renal injury may not work normally in pups. Along with upregulation of CYP24A1 in offspring kidneys exposed to maternal PM2.5, disrupted vitamin D signaling would affect the homeostasis of diverse cellular processes. Tight regulation of the RAS pathway during kidney development may also be impaired by vitamin D disturbance in dams and pups. It is unclear why intrarenal renin and ACE were suppressed in the pups of PM2.5-treated dams despite the suppression of VDR. It is also possible that the bradykinin system, circulating RAS, or another RAS component such as angiotensin 1 to 7 may be involved in intrarenal RAS imbalance in pups. Since proper RAS activation is necessary for normal pregnancy [10] and nephrogenesis [12], the suppression of the RAS in both dams and pups may negatively impact neonatal kidney development. Consistent with our findings, pups from dams that received AT1R blocker during lactation exhibited abnormal renal development that caused progressive renal deterioration [33]. Therefore, maternal PM2.5 exposure during nephrogenesis may disrupt intrarenal homeostasis of vitamin D metabolism and RAS in both dams and pups, leading to transgenerational renal impairment in rats. Aberrant suppression of the RAS might be transgenerational. While the opposite expression of VDR was found in dam and pup kidneys, the increases in oxidative damage and inflammation were shown in both dam and pup kidneys in parallel with glomerular and tubulointerstitial injury. In spite of the protective roles of VDR and klotho, nonresolving inflammation can initiate the pathogenic cascade, which eventually leads to the destruction of renal parenchyma. In this scenario, renal tissue remodeling process involved in the activation of VDR and klotho may occur simultaneously with the stimulation of inflammatory pathways in dam kidneys.
Oxidative stress and inflammation have been identified as the predominant underlying mechanisms for PM2.5-associated kidney damage in animal models [8]. Nrf2 is a cardinal regulator of antioxidant responses [34], and NF-κB is a key transcriptional factor of inflammatory gene expression [35]. The Nrf2 and NF-κB signaling pathways are assumed to interplay in the regulation of downstream target gene transcription [36]. Inhibition of NF-κB activation promoted TNF-α-induced generation of reactive oxygen species, lipid peroxidation, and protein oxidation [37]. The p50 subunit of NF-κB an important inflammatory response repressor, and animal studies suggest that the absence of p50 leads to significant increases in various pro-inflammatory mediators and severe unresolved inflammation [38]. In our study, PM2.5 exposure to pregnant and lactating dams induced kidney injury associated with oxidative stress and inflammatory response in both dams and pups. Maternal exposure to PM2.5 reduced intrarenal expressions of Nrf2 and NF-κB p50 in dams and pups while increasing TNF-α expression in dam kidneys. Dams and pups also exhibited increased renal cortical macrophage infiltration by PM2.5 exposure, which were lessened by vitamin D. Maternal vitamin D intake attenuated intrarenal TNF-α activity in dams and restored NF-κB p50 in pups at the end of lactation. As PM2.5 affects kidney injury through RAS activation [7], the activation of VDR and klotho and loss of renin in dam kidneys exposed to PM2.5 appear to be incompatible with increased inflammation and/or oxidative stress. However, as mentioned earlier, the protective mechanisms mediated by VDR and klotho against kidney injury in dams may be accompanied by downregulation of Nrf2 and NF-κB p50 and upregulation of TNF-α, because vitamin D supplementation effectively abrogated renal expressions of VDR, klotho, and TNF-α. Overexpression of VDR in cultured renal tubular cells consistently inhibited the induction of epithelial-to-mesenchymal transition, a key event in renal fibrogenesis, by TNF-α and transforming growth factor-β1 [32]. Here, the activation of VDR and klotho in dams exposed to PM2.5 could be early protective responses to renal inflammation and oxidative damage. However, concomitant intrarenal renin suppression during fetal nephrogenesis would affect the integrity of the systemic and placental RAS necessary for healthy pregnancy outcome. Neonatal suppression of intrarenal RAS may also lead to lifelong renal impairment and impaired nephrogenesis in developing kidney [33]. In Marin et al. [33], pups exposed to the AT1R blocker during lactation showed renal structural and functional disturbances persisting into adulthood.
During nephrogenesis, exposure to suboptimal intrauterine environments results in lifelong adverse consequences on renal structure and function. PM2.5 crosses the blood-placental barrier and circulates into fetal blood circulation, thereby acting as an in-utero environmental toxin [39]. Our findings reveal that PM2.5 exposure during the nephrogenic period causes renal injury in not only lactating mothers but also their offspring. Exposure to maternal PM2.5 induced renal disturbances in vitamin D signaling and RAS pathway that were associated with oxidative stress and inflammation in both exposed dam and offspring kidneys. Maternal vitamin D intake attenuated PM2.5-induced renal damage in dams and pups, reversing intrarenal VDR activity. Intrarenal ACE depletion in pup kidney was also restored by maternal vitamin D supplementation. Future studies should assess the benefits of vitamin D administration on renal outcomes linked to PM2.5 exposure. The effects of PM2.5 exposure on kidney might not be applied uniformly since the chemical composition and sources of PM2.5 change with different space and time. However, organic carbon and water-soluble ions of nitrate, sulfate, and ammonium were the major components of PM2.5 in this study, making up about 50% of PM2.5 mass [40]. In this study, the fine PM was orally administered via an orogastric tube in spite of air pollution. While there can be some differences in PM2.5 absorption through the lungs or the gastrointestinal tract, fine PM can eventually enter the systemic circulation and reach the placenta. In addition, the short-term exposure to PM2.5 during the second half of pregnancy and lactation in this experimental model may not be fully pertinent to the long-term renal impacts of exposure to PM2.5 during the entire pregnancy and lactation in human. However, our findings could be almost comparable to those of fetal exposure to PM2.5 during human nephrogenesis as the exposure time of PM2.5 in dams in our study corresponds to the period of rat nephrogenesis. There is still a need for additional investigations to advance our currently limited understanding of the association between PM exposure and adverse renal effects.
In this study, maternal exposure to fine PM during pregnancy and lactation induced renal impairment in both rat dams and their male offspring, as characterized by glomerular and tubulointerstitial injury, imbalance in the vitamin D signaling, aberrant RAS suppression, and increase in oxidative damage and inflammation. Renal deterioration was lessened by maternal vitamin D supplementation during the nephrogenic period. Kidney development could be affected by maternal PM2.5 exposure and maternal vitamin D intake may play a protective role against PM2.5-induced renal injury. The mechanism by which PM2.5 affects the RAS and vitamin D pathway still merits further research.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (Ministry of Science and ICT) (No. NRF-2020R1F1A1049554) and a grant from the Korean Nephrology Research Foundation (Pediatric Nephrology Research Fund, 2022).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: MHS, EP, HEY, YJN
Methodology: HEY, YJN, YSL, EKC, SHJ, JHL
Writing–original draft: MHS, EP, HEY
Writing–review & editing: All authors
All authors read and approved the final manuscript.


