Mortality associated with the neutrophil-lymphocyte ratio in septic acute kidney injury requiring continuous renal replacement therapy
Article information
Abstract
Background
Sepsis is an important cause of acute kidney injury in intensive care unit patients, accounting for 15% to 20% of renal replacement therapy prescriptions. The neutrophil-lymphocyte ratio (NLR), a marker of systemic inflammation and immune response, was previously associated with the mortality rate in multiple conditions. Herein, we aimed to examine how the NLR relates to the mortality rate in septic acute kidney injury patients requiring continuous renal replacement therapy (CRRT).
Methods
The NLRs of 6 and 18 were used for dividing NLRs into three groups and, thus, were set higher than those in previous studies accounting for steroid use in sepsis. Cox proportional hazard models were used to calculate hazard ratios of mortality outcomes before and after matching their propensity scores.
Results
A total of 798 septic acute kidney injury patients requiring CRRT were classified into three NLR groups (low, <6 [n = 277]; medium, ≥6 and <18 [n = 115], and high, ≥18 [n = 406], respectively). The in-hospital mortality rates per group were 83.4%, 74.8%, and 70.4%, respectively (p < 0.001). Per the univariable Cox survival analysis after propensity score matching, a high NLR was related to approximately 24% reduced mortality. The survival benefit of the high NLR group compared with the other two groups remained consistent across all subgroups, showing any p for interactions of >0.05.
Conclusion
A high NLR is associated with better clinical outcomes, such as low mortality, in septic acute kidney injury patients undergoing CRRT.
Introduction
Acute kidney injury (AKI) is a pivotal factor of increased mortality among critically ill patients admitted to the intensive care unit (ICU) [1–3]. Continuous renal replacement therapy (CRRT) is a rescue treatment option for patients with unstable vital signs and severe AKI. The number of severe AKI cases requiring CRRT has increased to more than 150,000 over several decades in the United States [4]. Despite advances in CRRT, its clinical outcomes owing to AKI remain negative [3,5,6]. Even though guidelines exist for CRRT implementation [7–9], CRRT-associated complications can occur, and performing CRRT does not always guarantee a survival benefit [10–12].
Sepsis is one of the most important causes of AKI in ICU patients, accounting for 15% to 20% of renal replacement therapy prescriptions [13,14]. Septic AKI has been associated with short-term mortality, subsequent progression to chronic kidney disease, end-stage renal disease, and increased long-term mortality [15,16]. Unfortunately, most patients with septic AKI are accompanied by many other risk factors [13,14], which make accurate and timely diagnosis difficult. Therefore, the ability to assess the severity of sepsis and initiate CRRT at an optimal time is important for improving survival in patients with septic AKI.
The neutrophil-lymphocyte ratio (NLR), a maker of systemic inflammation and immune response, has been actively studied worldwide to elucidate its diagnostic and prognostic efficacy in various disease conditions including malignancy, cardiovascular disease, diabetes, and autoimmune disease [17–20]. Also, NLR has been suggested as a useful marker of AKI and adverse clinical outcomes in AKI patients [21,22]. However, the association between NLR and clinical outcomes in septic AKI, particularly in those who require CRRT, is quite limited. Herein, we aimed to examine how NLR is associated with mortality outcomes in patients with septic AKI requiring CRRT.
Methods
Patients and data collection
This research was designed as a retrospective, observational, and single-center study. It was approved by the Institutional Review Board of Seoul National University Hospital (No. H-2110-085-1262) and complied with the Declaration of Helsinki. The requirement for informed consent was waived under approval.
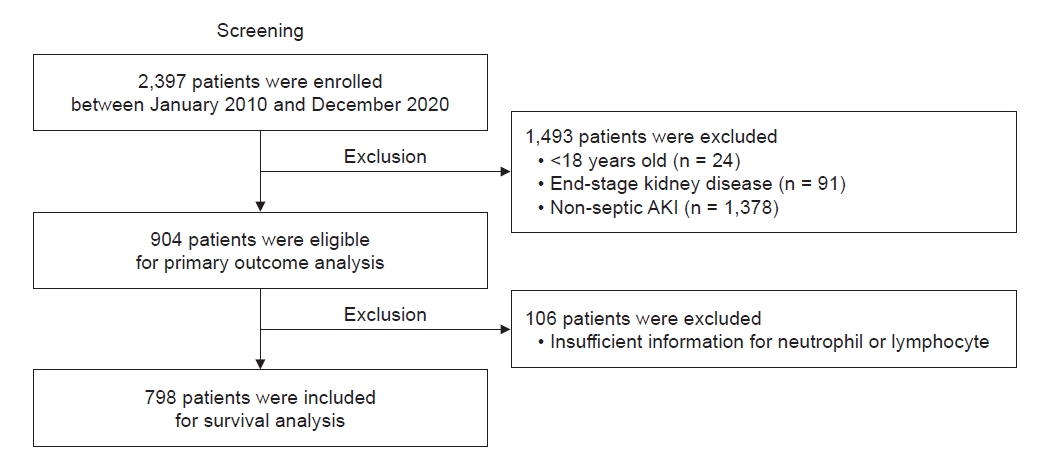
A total of 2,397 patients undergoing CRRT because of severe AKI were retrospectively reviewed at the Seoul National University Hospital from June 2010 to December 2020. In this study, we excluded patients aged <18 years (n = 24) and with a diagnosis of end-stage kidney disease (n = 91) or without a diagnosis of sepsis (n = 1,378) at the time of starting CRRT. Although 904 patients were eligible for the primary outcome analysis, patients without sufficient information about the neutrophil and lymphocyte count (n = 106) were excluded.
Baseline data, such as age, sex, weight, ICU division, use of inotropes, application of mechanical ventilator, type of central catheter, setting of CRRT (e.g., blood flow rate, target dose, and ultrafiltration), presence of anuria, and blood cell counts, were collected. Illness severity was assessed using the Charlson Comorbidity Index (CCI) [23], Sequential Organ Failure Assessment (SOFA) [24], and Acute Physiology Assessment and Chronic Health Evaluation (APACHE) II [25]. We retrieved the results of blood cell counts measured within 48 hours before starting CRRT; the result measured at the point closest to the start of CRRT was used for NLR calculation.
Since steroid use can affect not only the blood counts of neutrophils and lymphocytes but also their ratios [26,27], information on steroid use was collected based on the equivalent dose of intravenous (IV) hydrocortisone [28]. The primary outcome of this study was in-hospital mortality after starting CRRT.
Diagnostic criteria for infections
Septic AKI was defined as the occurrence of AKI within 7 days of sepsis diagnosis. AKI and sepsis were diagnosed according to Kidney Disease Improving Global Outcome (KIDIGO) criteria and Sepsis-3 criteria, respectively. The primary care physicians clinically diagnosed the infections, and then the definition of infection was referenced in the International Sepsis Forum Consensus Conference guidelines since 2005 [29]. The diagnosis of pneumonia required high clinical suspicion, including radiographic infiltration, fever or hypothermia, leukocytosis or leukopenia, and purulent respiratory secretions. Urinary tract infection was diagnosed based on typical symptoms and signs such as fever, dysuria, urgency, frequency, suprapubic tenderness, pyuria, bacteriuria, and suggestive imaging. Urine cultures were considered positive with the isolation of >105 colony-forming units (CFU)/mL of microorganisms (or 103 CFU/mL in catheterized patients). Intraabdominal infections included intraabdominal abscesses, peritonitis, biliary tract infections, pancreatic infections, enteritis, and colitis. Skin and soft tissue infections included cellulitis, necrotizing fasciitis, cutaneous gangrene, and surgical site infections. Infective endocarditis was diagnosed adherent to the revised Duke criteria. Patients were regarded as culture-positive if etiologic pathogens were obtained from blood or pleural fluid. In other cases, patients were considered culture-positive if semiquantitative cultures of sputum, blind endotracheal aspirates, or bronchoalveolar lavage detected moderate to heavy growths of bacteria with few epithelial cells on Gram stain examination (≤10 per high-power field). In diagnosing bacteremia, common contaminating organisms such as coagulase-negative staphylococci, Bacillus species, Corynebacterium species, micrococci, and Propionibacterium species were ignored unless considered clinically important by the primary care physicians or cultured from two or more blood sets. In this study, the diagnosis of culture-positive catheter-related bacteremia required a positive peripheral blood culture, while culture-negative catheter-related bacteremia was diagnosed clinically in the presence of purulent discharge, exit site, or tunnel tract infection.
Clinical management
Treatment of patients in the ICU was at the physician’s discretion, and physicians were recommended to follow the guidelines of the up-latest Survival Sepsis Campaign [30,31]. Although the treatments were not protocolized, they included active fluid resuscitation and vasopressors with hemodynamic data obtained via lactate and N-terminal B-type natriuretic peptide measurements, transthoracic echocardiography, and arterial pressure waveform analyses when indicated. An early intubation strategy was encouraged in impending respiratory failure. Blood cultures were drawn early with 20 mL of blood equally injected for each set of aerobic and anaerobic media, while cultures of other sites were performed based on the focus of infection. Empirical broad-spectrum antibiotics were chosen considering the suspected pathogen and were optimized and/or de-escalated according to the culture results.
The implementation of CRRT because of severe AKI was adherent to the KDIGO guideline [8]. To deliver 20 to 25 mL/kg/hour, we conducted CRRT with a target dose of 30 to 35 mL/kg/hour in accordance with the KDIGO guideline [8], and for some patients with acute respiratory distress syndrome, the target dose was raised up to 40 mL/kg/hour.
Statistical analysis
Baseline characteristics are described as proportions and means ± standard deviations when categorical and continuous variables are normally distributed and as medians with interquartile ranges when they are not normally distributed. The normality of the distribution was analyzed using the Kolmogorov-Smirnov test. The chi-square test or Fisher exact test was employed to compare categorical variables. The Student t test or Mann-Whitney U test was used for continuous variables with or without a normal distribution, respectively.
We determined NLRs 6 and 18 as the criteria for dividing NLRs into three groups based on the tertiles of the NLR values observed within our study cohort. All statistical analyses were performed on these three NLR groups. The criteria of 6 and 18 were set slightly higher than those in previous studies, considering that steroids are commonly used in sepsis and reactive neutrophilia accompanied by lymphopenia is also common. We tested the proportional hazard assumption using the Schoenfeld test. The Kaplan-Meier survival curves were drawn, and differences in the curves were determined using a log-rank test. Because many baseline parameters differed between groups, propensity score-based matching with inverse probability treatment weighting was additionally performed. All baseline variables, such as age, sex, weight, ICU division, use of inotropes, application of mechanical ventilator, type of central catheter, blood flow rate, target dose, target ultrafiltration, presence of anuria, CCI, SOFA score, APACHE II score, and IV hydrocortisone equivalent dose, were included for calculating propensity scores. Finally, a two-way analysis of variance was performed to evaluate the effect modification of NLR on the all-cause mortality in each subgroup. A two-tailed p-value of <0.05 was considered statistically significant. All statistical analyses were performed using R software (version 4.1.2; R Foundation for Statistical Computing).
Results
Data collection and baseline characteristics
A total of 798 patients with septic AKI requiring CRRT were included for survival analysis (Fig. 1), and they were classified into three NLR groups: NLR of <6, ≥6 and <18, and ≥18 (277, 115, and 406 patients, respectively) (Table 1). Among the 798 participants, the mean age was 64.2 ± 14.5 years, and 63.0% of the patients were male. Moreover, 58.4% of the patients were hospitalized in the medical ICU. Half of the patients used inotropes, and approximately 80% were supported by mechanical ventilation. The mean SOFA and APACHE II scores were 12.8 ± 3.7 and 27.4 ± 7.7, respectively. Both SOFA and APACHE II scores were highest in the group with NLR ≥18, with 13.7 ± 3.3 and 27.8 ± 7.4, respectively. Among all participants, 67.2% used steroids at least once. The group with NLR <6 had the highest percentage of steroid-using patients and the highest daily dose of IV hydrocortisone equivalent, with 81.9% and 200 mg, respectively, whereas the group with NLR ≥6 and <18 had the lowest percentage. While the percentage of patients with bacteremia confirmed by positive blood cultures did not differ significantly amongst the three NLR groups, it is interesting to note that there were some distinct patterns in the infection sources across each NLR group. In the group with NLR <6, there was a higher incidence of infections linked to features of hematologic, oncologic, and immunocompromised conditions, including instances of neutropenic septic shock. On the other hand, the group with NLR >18 demonstrated the highest prevalence of pneumonia and intraabdominal infections. Significant differences were noted between the three groups regarding the ICU division, SOFA score, underlying diabetes mellitus, steroid use, IV hydrocortisone equivalent dose, and infection source. The baseline characteristics of each group after propensity score-based matching with all variables are shown in Supplementary Table 1 (available online).
Association between neutrophil-lymphocyte ratio and survival
During a median follow-up period of 10 days (interquartile range, 3–28 days), 603 patients (75.6%) died. The incidence of mortality was 29.4 deaths per 1,000 person-days (Table 2). The in-hospital mortality rates of the groups with NLR <6, ≥6 and <18, and ≥18 were 83.4%, 74.8%, and 70.4%, respectively (p < 0.001). All kinds of mortality rates were better in the group with NLR ≥18 than in the other two groups. The survival curves of the three groups revealed significantly different curve trends (p < 0.001) and the survival probability was higher in the group with NLR ≥18 than in the other two groups (Fig. 2A). When all variables were adjusted in model 4, the group with NLR ≥18 was associated with a 26.4% reduction in mortality based on hazard ratio values, compared with the group with NLR <6 (Table 3). However, there was no significant difference in mortality rates between the group with NLR <6 and the group with NLR ≥6 and <18 in the fully adjusted model 4. In the univariable Cox survival analysis, performed after propensity score matching for all variables, the group with NLR ≥18 demonstrated an approximately 24% reduction in mortality based on hazard ratio values, compared with the group with NLR <6 (Table 4). Similar to the result of the fully adjusted model 4 (Table 3), no significant difference in mortality rates between the group with NLR <6 and the group with NLR ≥6 and <18 was noted even after propensity score matching. The survival curves of the three groups after matching methods showed that the curve trends remained different from each other (p < 0.001) (Fig. 2B), while the survival curve of the group with NLR ≥6 and <18 was closer to that of the group with NLR <6 after propensity score matching. In the subgroup analysis, the survival benefit of the group with NLR ≥18 compared with that of the other two groups remained consistent across all subgroups, such as age, sex, ICU division, SOFA score, APACHE II score, and steroid use, showing any p for interactions of >0.05 (Fig. 3).
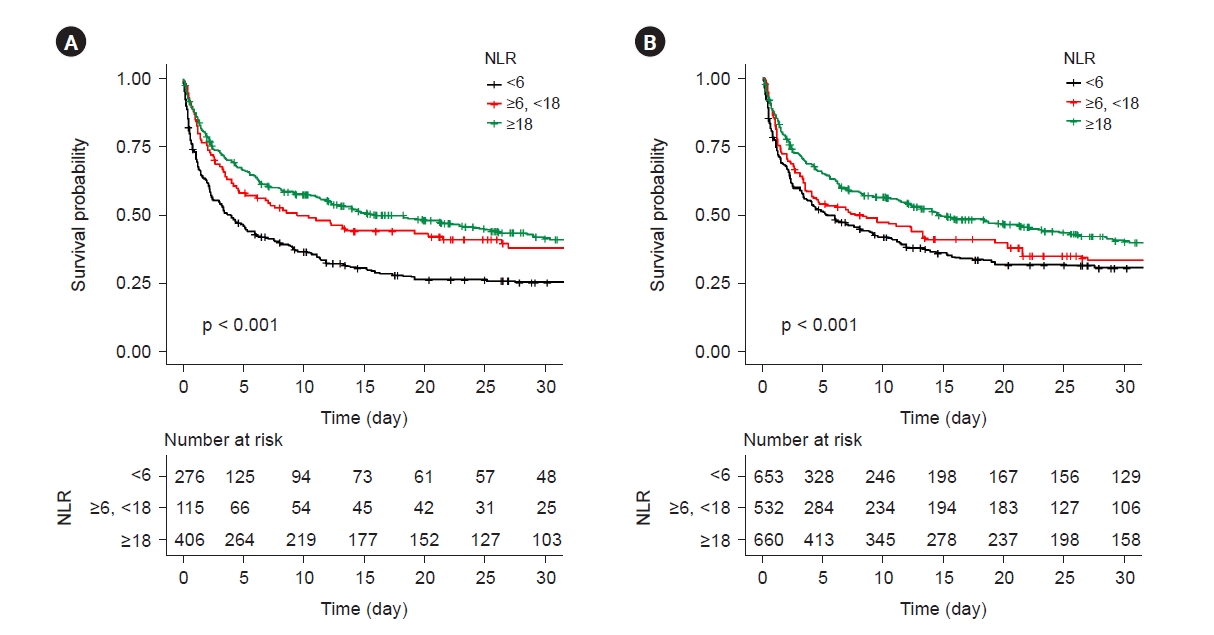
Kaplan-Meier survival curves.
(A) Kaplan-Meier survival curves of three different neutrophil-lymphocyte ratio (NLR) tertile groups before propensity score matching. (B) Kaplan-Meier survival curves of three different NLR tertial groups after propensity score matching.
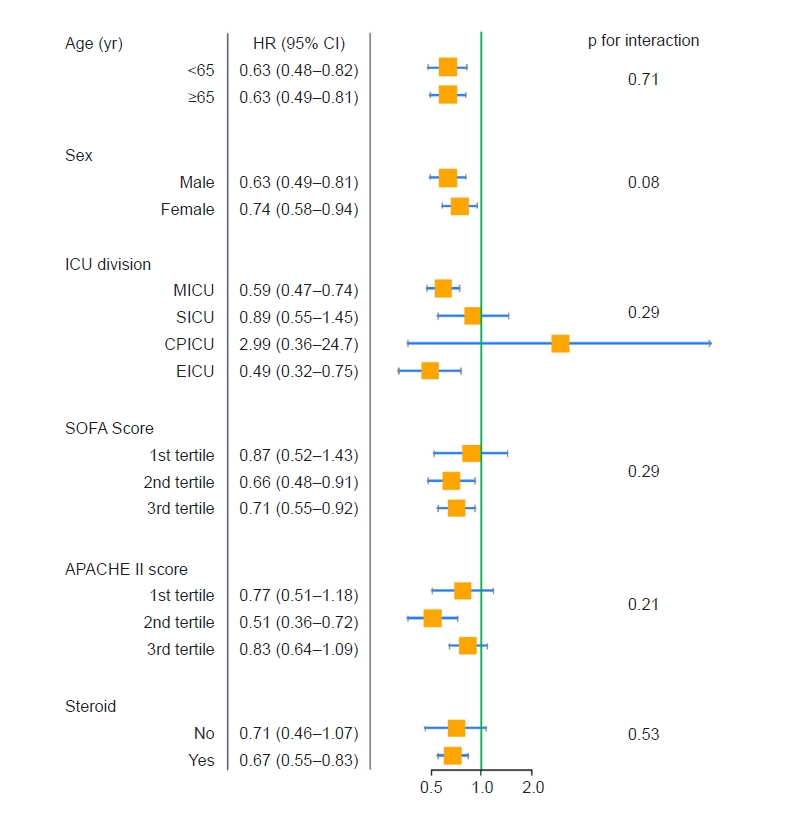
Forest plot for subgroup analysis.
Event is all-cause mortality and 1st + 2nd NLR tertiles are reference (vs. 3rd NLR tertile). APACHE, acute physiologic and chronic health evaluation; CI, confidence interval; CPICU, cardio-pulmonary intensive care unit; EICU, emergency intensive care unit; HR, hazard ratio; ICU, intensive care unit; MICU, medical intensive care unit; NLR, neutrophil-lymphocyte ratio; SICU, surgical intensive care unit; SOFA, Sequential Organ Failure Assessment.
Discussion
Because patients with septic AKI who require CRRT are in a critical condition [13,14], clinicians should consider the patient’s status comprehensively, including vital signs, biochemical results, imaging tests, and medical history. Among these factors, changes in circulating white blood cells, particularly neutrophilia with relative lymphocytopenia, are known to be associated with the degree of systemic inflammatory response [17–20]. Moreover, because routine hematological tests, such as complete blood count, are performed regularly for patients admitted to ICU, NLR is easy to calculate and apply in real-world practice.
In this study, we identified that a higher NLR was associated with a lower mortality rate, even after propensity score matching and adjustment for the IV hydrocortisone equivalent dose in the multivariable Cox survival analysis. This is inconsistent with many previous studies dealing with various diseases such as cancer, cardiovascular disease, autoimmune disease, and infection. In those studies, a high NLR was associated with poor prognosis and showed predictive power in diagnosing diseases [17–20]. For instance, Sarraf et al. [17] reported that increasing preoperative NLRs were associated with a more advanced cancer stage but remained an independent predictor of survival after complete resection for primary lung cancer. Moreover, Huang et al. [18] stated that an increased NLR was significantly associated with diabetic nephropathy, and high NLR values might be a reliable predictive marker of early-stage diabetic nephropathy. In a meta-analysis performed on systemic lupus erythematosus (SLE), NLR was significantly higher in patients with SLE compared with healthy controls and was positively correlated with the SLE disease activity index, suggesting that NLRs can be useful biomarkers in the management of SLE [19]. Even in the context of infections like sepsis, elevated NLRs were observed in the early phase of sepsis, and NLRs could be helpful biomarker in predicting the prognosis and be employed to discontinue antimicrobials [20].
Several previous studies that explored the relationship between NLR and AKI showed a prevailing trend tchat higher NLRs with unfavorable outcomes in AKI patients [21,22,32]. Chen et al. [21] presented a J-shaped relationship between NLR and a composite outcome including stage 3 AKI, the need for dialysis, or 14-day in-hospital mortality with the lowest odds ratio observed for an NLR between 7 and 38. Further, Wei et al. [22] highlighted that an NLR exceeding 8.69 upon hospital admission corresponded to increased mortality and disease severity in septic AKI patients. Similarly, Gameiro et al. [32] revealed that an elevated neutrophils to lymphocytes and platelets (N/LP) ratio upon ICU admission was an independent risk factor for in-hospital mortality among septic AKI patients. This study included patients with septic AKI admitted to the ICU with or without dialysis and showed that mortality was particularly high in patients with N/LP >14, but the area under the curve for predicting mortality in septic AKI was relatively low at 0.565 [32]. These results suggest that higher NLR is primarily associated with poorer prognosis in AKI patients [21,22,32]. In this cohort of patients with septic AKI who received CRRT, a higher NLR before CRRT initiation was associated with a higher survival rate, which is contrary to the previous study results [17–22,32]. These conflicting findings are likely due to the different time points for measuring NLR in each study, different inclusion criteria of the study population, and institutional-specific factors like clinical practice and patient features.
There have been several experimental rationales that CRRT has an advantage in removing cytokines and endotoxins [33,34]. In addition, it is well established that endotoxins and cytokines such as interleukin (IL) 1, IL-6, and tumor necrosis factor alpha are associated with biochemical markers such as C-reactive protein and procalcitonin [35], as well as clinical features such as mortality, hospitalization, and hospital length of stay [36]. Although all subjects enrolled in this study were classified as septic AKI, the actual etiology of AKI was not solely due to sepsis. Therefore, in the high-NLR status, the burden of cytokines and endotoxins would be relatively higher, and CRRT might have a more beneficial effect on clinical outcomes by removing larger amounts of cytokines and endotoxins.
To date, there have been several observational retrospective trials showing that early CRRT results in increased survival of patients with severe septic AKI [37,38]. The beneficial effect of early CRRT initiation was inconsistently found in randomized controlled trials [35,39]. In most of those studies, serum creatinine elevation and oliguria were used as the criteria for early initiation of CRRT. However, in early-stage AKI, a nearly 50% reduction in creatinine clearance is required for a significant increase in the serum creatinine concentration [40]. Therefore, the serum creatinine concentration would be inappropriate as a sensitive indicator for diagnosing early-stage AKI. We suggest that monitoring the NLR alongside oliguria and azotemia could be helpful in predicting the prognosis of patients with severe septic AKI when contemplating the implementation of CRRT.
The strengths of the present study include robust statistical analysis without missing values. Additionally, although baseline SOFA and APACHE II scores were higher in the group with a higher NLR, they showed better survival outcomes, strongly supporting the hypothesis of this study. However, there are several limitations to be discussed. Since the study design was retrospective, the results could not imply causality between high NLRs and survival outcomes. Even though we used matching techniques to overcome selection bias and other problems, they might still have persisted. Specifically, the association between changing patterns of NLRs and survival outcomes remains unknown, as the NLR was not continuously measured after CRRT initiation. Because we did not measure the amount of cytokines or endotoxins removed before and after CRRT, it remains unclear whether the high survival rate associated with a high NLR is a consequence of more cytokine and endotoxin removal. Also, even though steroid use was adjusted with an IV hydrocortisone equivalent dose, the effect of steroids on the NLR could not be completely controlled. Finally, there is an important consideration that the lowest NLR group had a significantly higher proportion of patients on immunosuppressants or chemotherapy, which may have affected our results.
In conclusion, a high NLR (≥18) is associated with better clinical outcomes, such as low mortality, particularly in septic AKI patients requiring CRRT. Although we do not directly advocate for initiating CRRT based solely on NLR values from our findings, we believe that these associations offer valuable insights into the potential clinical utility of NLR. We eagerly anticipate further studies on NLR to explore potential causal relationships and determine the best strategies for employing NLR to enhance patient outcomes.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.23.116).
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Data sharing statement
The datasets used and analyzed during the current study are available from the corresponding author upon reasonable request.
Authors’ contributions
Conceptualization, Methodology: JL, JS, YCK
Formal analysis: JL, JS, SGK, DY, MWK
Investigation: DKK, KHO, KWJ, YSK, SSH, YCK
Writing–original draft: JL, JS, YCK
Writing–review & editing: SSH, YCK
All authors read and approved the final manuscript.