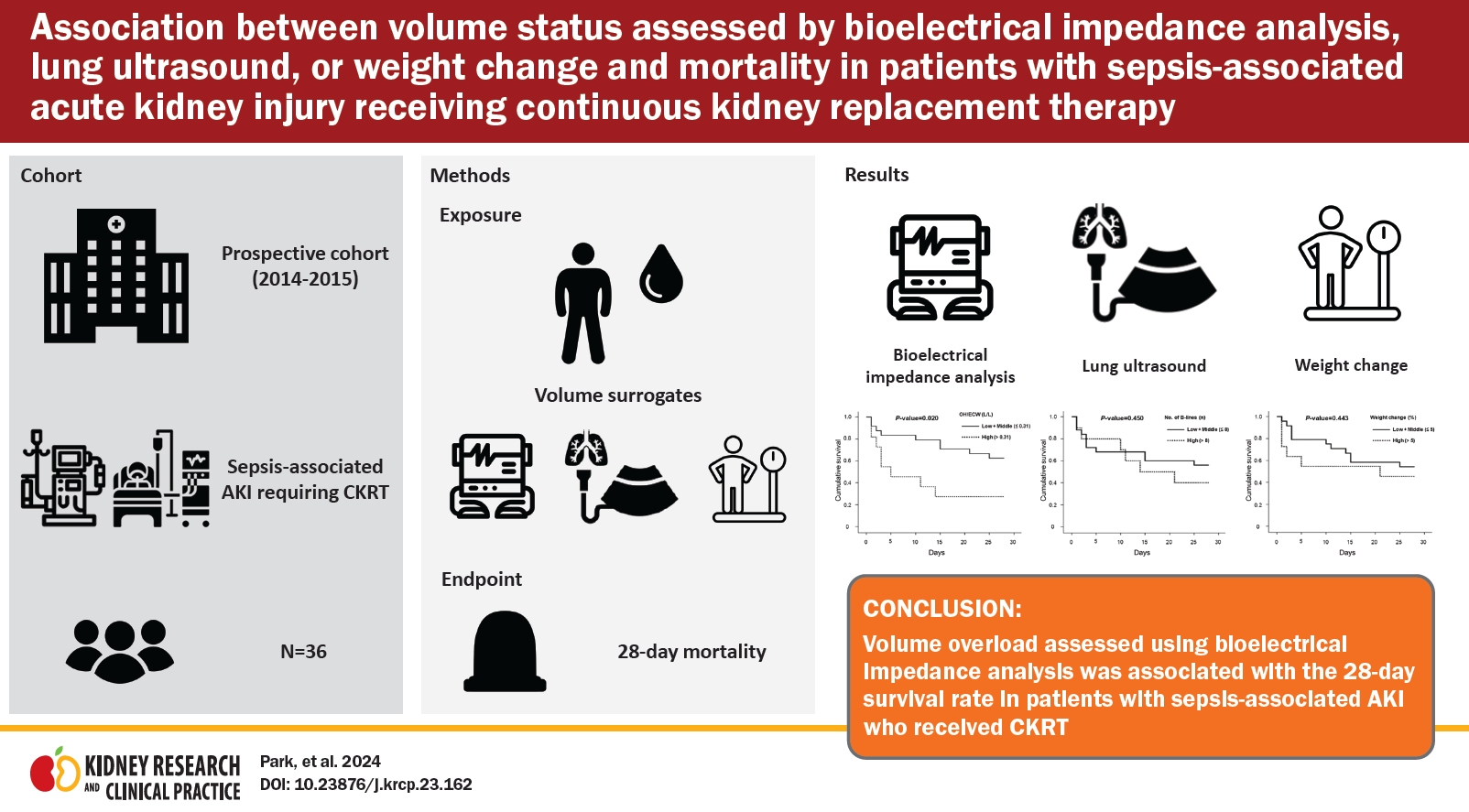
Association between volume status assessed by bioelectrical impedance analysis, lung ultrasound, or weight change and mortality in patients with sepsis-associated acute kidney injury receiving continuous kidney replacement therapy
Article information
Abstract
Background
Fluid overload is an independent risk factor of mortality in patients with acute kidney injury (AKI) receiving continuous kidney replacement therapy (CKRT). However, the association between fluid status, as assessed by bioelectrical impedance analysis (BIA) or lung ultrasound, and survival in patients with AKI requiring CKRT has not been established.
Methods
We analyzed 36 participants with sepsis-associated AKI who received CKRT at a tertiary hospital. The main exposures were volume surrogates: 1) overhydration normalized by extracellular water (OH/ECW, L/L) assessed by BIA, 2) the number of B-lines measured by lung ultrasound, and 3) weight change ([body weight at CKRT initiation – body weight at admission] × 100/body weight at admission). The primary outcome was the 28-day mortality.
Results
Seventeen participants (47.2%) died within 28 days. There were no significant correlations between OH/ECW and weight change (R2 = 0.040, p = 0.24), number of B-lines and OH/ECW (R2 = 0.056, p = 0.16), or weight change and number of B-lines (R2 = 0.014, p = 0.49). Kaplan-Meier analyses revealed that patients in the highest tertile of OH/ECW showed a significantly lower cumulative 28-day survival probability than the others (the lowest + middle tertiles). The survival probability of participants in the highest tertile of the number of B-lines or weight change did not differ from that of their counterparts. In a multivariate Cox proportional hazard model, the hazard ratio for the highest tertile of OH/ECW was 3.83 (95% confidence interval, 1.04–14.03).
Conclusion
Volume overload assessed using BIA (OH/ECW) was associated with the 28-day survival rate in patients with sepsis-associated AKI who received CKRT.
Introduction
Acute kidney injury (AKI) is a frequent and serious complication among critically ill patients treated in intensive care units (ICUs) [1,2]. It has been reported that AKI increases the risk of death to approximately 60% to 80% [2–4]. Sepsis is the most common cause of AKI in patients admitted to ICUs [5,6]. Patients with sepsis-associated AKI often require kidney replacement therapy (KRT), and continuous KRT (CKRT) is the preferred modality for those patients [7,8].
Patients with sepsis-associated AKI who required CKRT had the highest risk of mortality [9,10]. Accordingly, several studies have been conducted to identify risk factors that may independently influence clinical outcomes in those patients. Recently, the fluid status of patients with sepsis-associated AKI receiving CKRT, as assessed by body weight change or cumulative fluid balance, has been suggested as an independent risk factor for survival [11–13].
Critically ill patients with AKI are often complicated by fluid overload, and accurate assessment of fluid status is essential for the appropriate management of those patients [11,14]. Bioelectrical impedance analysis (BIA) and lung ultrasound have recently been introduced to evaluate patients’ fluid status [15–18]. BIA estimates a patient’s body composition using electrical resistance, and the number of B-lines acquired via lung ultrasound confers information on the water content in the lungs [15,18]. However, the association between fluid status evaluated using BIA or lung ultrasound and survival in patients with sepsis-associated AKI receiving CKRT has rarely been investigated.
Therefore, we examined the association between fluid status evaluated using BIA or lung ultrasound and mortality in patients with sepsis-associated AKI receiving CKRT.
Methods
Study design and participants
This prospective, observational study was conducted at a tertiary care hospital (Severance Hospital, Seoul, Republic of Korea) between April 2014 and February 2015. This study was approved by the Institutional Review Board of Severance Hospital and was conducted in accordance with the provisions of the Declaration of Helsinki (No. 4-2014-0791). All participants and/or substitute decision makers were informed of the study and provided written informed consent.
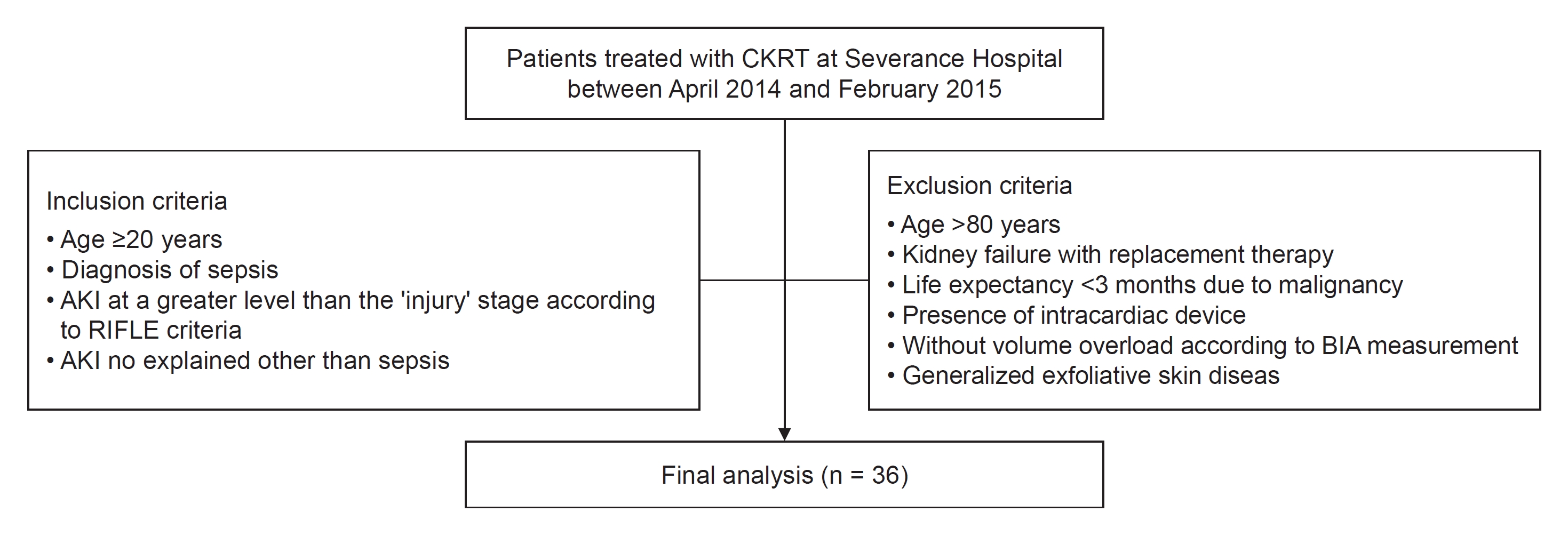
Participants were eligible for enrollment if they were 20 years or older and satisfied following three criteria: 1) diagnosis of sepsis according to consensus conference criteria suggested by the Society of Critical Care Medicine/American College of Chest Physicians [19]; 2) AKI at a greater level than the ‘injury’ stage according to the RIFLE (Risk, Injury, Failure, Loss of kidney function, and End-stage kidney disease) criteria [20]; and 3) AKI not explained other than sepsis. Briefly, if a patient had a suspected infection and met the criteria for systemic inflammatory response syndrome (two or more of the following: body temperature of <36 ℃ or >38 ℃, heart rate of >90 beats/min, respiratory rate of >20 breaths/min or PaCo2 <32 mmHg, or white blood cell count of <4.0 × 103 μL or >12.0 × 103/μL) in two consecutive measurements, the diagnosis of sepsis was established. Infection was diagnosed if the causative organisms were isolated by culture studies or was clinically suspected when patients satisfied one of the following criteria: 1) white blood cells in normally sterile fluid, 2) perforated viscus, or 3) infection focus detected on radiological examination. AKI at a greater level than the ‘injury’ stage was defined as a more than two-fold increase in serum creatinine level compared with baseline or urine output of <0.5 mg/kg/hr over 12 hours according to the RIFLE criteria. The exclusion criteria were as follows: 1) patients older than 80 years; 2) patients who had already been undergoing KRT due to kidney failure; 3) patients who had been diagnosed with malignancy and had a life expectancy of less than 3 months; 4) patients who had an intracardiac device, including a pacemaker, implantable cardioverter-defibrillator, or cardiac resynchronization therapy; 5) patients without volume overload according to BIA measurements; and 6) patients with generalized exfoliative skin disease. Finally, 36 participants were enrolled (Fig. 1).
Data collection and measurements
Demographic data, such as age, sex, body weight, and medical history, were collected from electronic medical records at the time of enrollment. Clinical and biochemical data were collected at CKRT initiation. The Acute Physiology and Chronic Health Evaluation (APACHE) II score was determined upon ICU admission. Serum creatinine levels were measured using an isotope-dilution mass spectrometry-tractable method. Body weights were measured using a scale. As a surrogate for volume status, we assessed weight change (%), defined as the difference in body weight at CKRT initiation from the body weight at the time of admission, which was normalized by the body weight at the time of admission. Weight change (%) was calculated using the following formula:
Bioelectrical impedance analysis measurement and lung ultrasound
The amount of fluid overload was assessed by BIA using the Body Composition Monitor (Fresenius Medical Care) according to the manufacturer’s instructions within 6 hours of CKRT initiation. Briefly, the participants were removed from the metallic devices or accessories. A pair of electrodes was placed on the dorsum of the hand and the foot on the ipsilateral side. Overhydration (OH) and extracellular water (ECW) were measured by BIA. Simultaneously, lung ultrasound was performed using a portable ultrasound scanner (GE Logiq Book XP; General Electric Health Care) with a 2 to 3.6 MHz phased array cardiac assessment EM 3S-RS probe. The number of B-lines was measured at four points on the thorax: the intercostal spaces between the third and fourth ribs and the sixth and seventh ribs on each midclavicular line. The numbers were recorded at each point and summed to obtain the number of B-lines. OH normalized by ECW (OH/ECW, L/L) and the number of B-lines acquired using lung ultrasound were adopted as surrogates for patient volume status.
Continuous kidney replacement therapy procedure
CKRT was initiated at the discretion of a consulting nephrologist without considering the patient’s eligibility for this study. Generally, CKRT is prescribed in patients with AKI at a stage greater than the injury stage classified by the RIFLE criteria, with the presence of significant volume overload, intractable hyperkalemia (potassium of >6.5 mEq/L), or severe acidemia (pH <7.2). Vascular access for CKRT was made in the internal jugular or femoral veins using a 14-French double-lumen catheter. CKRT was performed using Prismaflex machines (Gambro) with ST100 (surface area, 1.0 m2) filter sets. The effluent volume was set to achieve a clearance of 30 to 40 mL/kg/hr, with a blood flow rate of 150 to 200 mL/min. Changes in the maintenance of CKRT, blood flow rate, replacement fluid flow, or ultrafiltration rate in each patient were determined by both the consulting nephrologist and the attending physician.
Patients remained on CKRT until kidney function recovered, transferred to conventional hemodialysis, withdrew CKRT as part of life support, or died. The decision to wean patients from CKRT was first made by the nephrologist when the patient recovered hemodynamic stability for undergoing intermittent hemodialysis or had considerable urine output (>1,000 mL/day).
Exposure and outcome
The exposure of interest was the volume status of the participants. To measure the volume status, we used OH/ECW (L/L), number of B-lines, and weight change (%). Participants were classified according to tertiles of OH/ECW (L/L), number of B-lines, or weight change (%). The primary outcome was death from any cause within 28 days of CKRT initiation.
Statistical analysis
To investigate the association between surrogates for volume status and 28-day mortality in patients with sepsis-associated AKI receiving CKRT, we first compared survival among the groups (the lowest + middle tertiles vs. the highest tertile) using Kaplan-Meier analyses with the log-rank test. The Cox proportional hazards model was used to examine the association between a surrogate that showed a significant association with the primary outcome in the Kaplan-Meier analysis. We made incremental adjustments with the following variables: Model 1 is a crude model. Model 2 was adjusted for age, sex, diabetes, and chronic kidney disease. In Model 3, APACHE II score and total serum CO2 levels were added. The results of Cox proportional hazards regression are presented as hazard ratios (HRs) and 95% confidence intervals (CIs).
Data were analyzed using PASW Statistics version 18.0 (IBM Corp.). Statistical significance was set at p < 0.05.
Results
Baseline characteristics
Table 1 presents the baseline characteristics of the 36 participants according to tertiles of volume overload (OH/ECW). The mean age was 64.6 years (standard deviation, 14.6 years) and 61.1% were male . Overall, participants in the highest tertile of volume overload (OH/ECW) were more likely to have a higher left atrial volume index and APACHE II scores. There were no significant differences in other demographic factors, medical histories, or laboratory values between the tertiles. The baseline characteristics of the participants according to tertiles of volume overload estimated by the number of B-lines or weight changes are summarized in Supplementary Table 1, 2 (available online).
Correlations between volume overload surrogates
Supplementary Fig. 1 (available online) shows the correlations among the three markers of volume overload. OH/ECW, the number of B-lines, and weight change did not show significant correlations between OH/ECW and weight change (R2 = 0.040; p = 0.24), the number of B-lines and OH/ECW (R2 = 0.056; p = 0.16), or weight change and the number of B-lines (R2 = 0.014, p = 0.49).
Association between volume assessed by bioelectrical impedance analysis, lung ultrasound, or weight change and patient survival
A total of 17 patients (47.2%) died within 28 days of CKRT initiation. Kaplan-Meier curves revealed that the cumulative 28-day survival probability was significantly lower for patients in the highest tertile of OH/ECW compared to other tertiles (p = 0.02). However, the survival rate at 28 days after CKRT initiation did not significantly differ between groups classified according to the number of B-lines (p = 0.45) or weight change (p = 0.44) (Fig. 2).
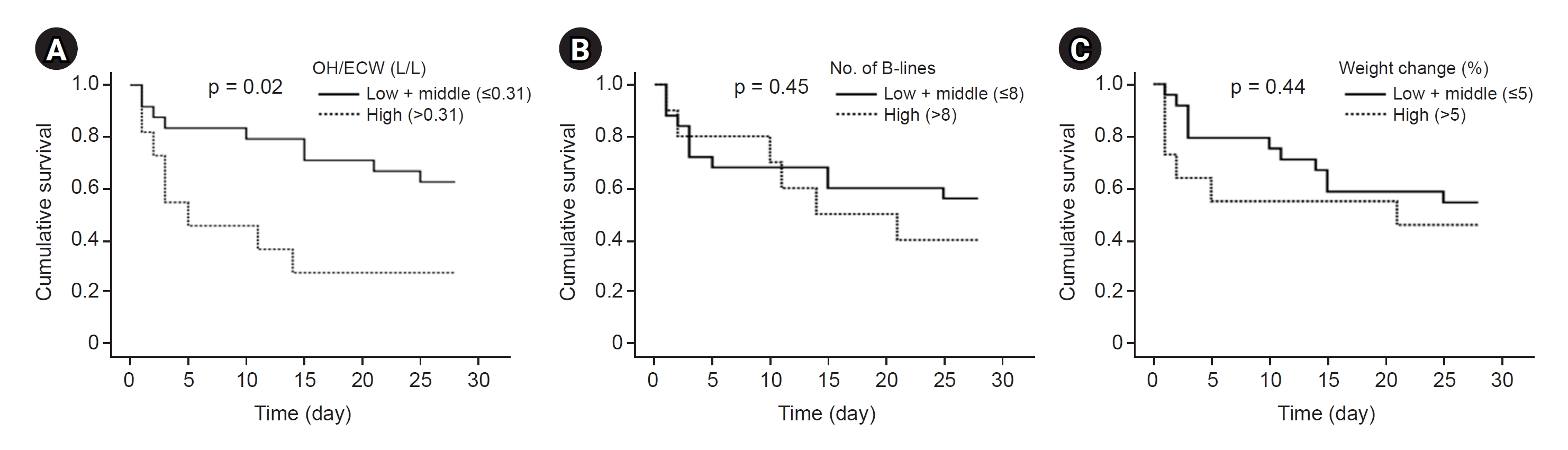
Kaplan-Meier curves showing patient 28-day survival.
Cumulative survival probability within 28 days according to overhydration (OH)/extracellular water (ECW) categories (A), the number of B-lines categories (B), and weight change categories (C). Log-rank tests were used for comparison between groups.
Next, the association between volume overload assessed using BIA (OH/ECW) and patient survival was evaluated using multivariate Cox proportional hazard models. In the unadjusted model, the HR for the risk of 28-day mortality was 2.93 (95% CI, 1.11–7.73) for the highest tertile compared with the lowest + middle tertiles (model 1 in Table 2). The association between OH/ECW and the primary outcome was maintained after adjusting for demographic factors and comorbidities (model 2 in Table 2). Further adjustment for the APACHE II score and serum total CO2 level did not change the increased risk in the highest tertile of OH/ECW. The corresponding HR for the highest tertile was 3.83 (95% CI, 1.04–14.03) (model 3 in Table 2).
Discussion
In this prospective observational study, we showed that volume status evaluated using BIA was significantly associated with 28-day survival in patients with sepsis-associated AKI receiving CKRT. We did not find a significant association between the volume status assessed using lung ultrasound or weight change and 28-day mortality in these patients. Our findings suggest that fluid overload at the time of CKRT initiation is associated with worse 28-day survival in patients with sepsis-associated AKI receiving CKRT. In addition, volume assessment using BIA was found to have prognostic value in predicting adverse outcomes in these patients.
Recent studies have revealed that the mortality rate observed in critically ill patients with AKI is >50%; poor survival has been attributed to multiple complications associated with AKI, such as fluid overload, electrolyte imbalance, bleeding, and infection [21,22]. Fluid overload often precedes and follows the diagnosis of AKI [23,24]. The impact of fluid overload on survival in AKI patients has been evaluated in several studies. Bouchard et al. [11] found that fluid overload, defined as >10% increase in body weight relative to baseline, was associated with mortality among critically ill patients with AKI in a multicenter observational study involving more than 600 patients in North America. In a Finnish AKI study involving critically ill patients receiving KRT (FINNAKI study), cumulative fluid accumulation was revealed to be an independent risk factor for 90-day mortality [25]. Additionally, Woodward et al. [26] found that the fluid balance from admission to CKRT initiation was associated with in-hospital mortality in critically ill patients with AKI requiring CKRT.
Accurate volume status assessment is considered a key element in the management of patients receiving CKRT and risk stratification of those patients [14,27]. Conventionally, body weight measurement, review of daily fluid balance, physical examination, and chest radiograph have been used to estimate fluid overload in those patients. However, these approaches typically yield inconsistent results and are considered to be fairly inaccurate [28–30]. Recently, several measures have been introduced to more precisely evaluate the volume status of patients [29]. Among these, BIA and lung ultrasound are simple and relatively reliable methods for estimating body fluid status, particularly for detecting fluid overload [15–18]. Nonetheless, information regarding the prognostic implications of volume status assessed by BIA or lung ultrasound in patients with AKI requiring CKRT is relatively scarce.
In the present study, we performed analyses to investigate the relationships between three volume surrogates (OH/ECW, number of B-lines, and weight change) and the association between volume surrogates and 28-day survival in patients with sepsis-associated AKI receiving CKRT. Our findings showed that the volume status assessed using various measures did not show a significant correlation. Moreover, participants with higher OH/ECW had a higher risk of 28-day mortality, whereas the number of B-lines or weight change was not significantly associated with 28-day mortality. In line with our findings, an ambispective cohort study including 152 patients with AKI treated with either intermittent hemodialysis or CKRT showed that OH/ECW before the initiation of KRT was significantly associated with patient survival [31]. However, this study did not collect information regarding volume status estimated by lung ultrasound or weight change; thus, they could not examine the relationship between surrogates for volume overload and the association between volume status surrogates and patient outcomes in the same cohort. Therefore, our results may provide novel insights into the measurement of volume status and the prognostic value of volume surrogates in patients with AKI requiring CKRT. In other words, the severity of fluid overload may be estimated differently depending on the methods employed, and volume assessment using BIA can provide additional information on patient prognosis.
Our study has several limitations. First, due to the observational nature of this study, the possibility of residual confounding factors cannot be excluded. However, after adjusting for various factors that may contribute to patient outcomes, we consistently found the association between volume status assessed via BIA and 28-day survival. Second, our study was conducted at a single center. Third, our results may be underpowered because of the relatively small number of participants. Fourth, our cohort consisted of only Korean participants, which limits the generalizability of the study. Thus, our study results cannot be directly extrapolated to patients with ethnic backgrounds other than Korean.
In conclusion, our study revealed that the extent of volume can vary significantly depending on the measurement methods used. Among the fluid overload surrogates investigated in this study, only OH/ECW was associated with 28-day survival in patients with sepsis-associated AKI receiving CKRT. Our findings require further validation in larger cohorts and well-designed clinical trials to determine the optimal ultrafiltration strategy to improve survival in patients with sepsis-associated AKI who require CKRT.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.23.162).
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This study was supported by a research fund by the Yonsei University College of Medicine. The sponsor had no role in the study design, data collection, or analysis.
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization: CHP, SGH, SJK, SWK
Data curation: CHP, SGH, HWK
Formal analysis, Visualization: CHP, SGH, HWK, SWK
Funding acquisition: SGH
Investigation, Methodology: CHP, SGH, JTP, SHH, SJK, SWK
Project administration, Resources, Supervision, Validation: JTP, SHH, SJK, SWK
Writing-original draft: CHP, SGH, SJK, SWK
Writing-review & editing: All authors
All authors read and approved the final manuscript.