Incident dementia in kidney transplantation recipients: a matched comparative nationwide cohort study in South Korea
Article information
Abstract
Background
Recent studies have shown that patients with end-stage renal disease (ESRD) are at elevated risk of dementia. However, whether kidney transplantation (KT) lowers the risk for incident dementia remains unclear.
Methods
From the Korean National Health Insurance Service database, we identified incident KT recipients aged ≥40 years without any history of dementia between 2007 and 2015. We also established a pair of age-, sex-, and inclusion year-matched control cohorts of patients with incident dialysis-dependent ESRD and members of the general population (GP) without a history of dementia, respectively. Cases of incident all-cause dementia, including Alzheimer disease (AD), vascular dementia (VD), and other kinds of dementia, were obtained from baseline until December 31, 2017.
Results
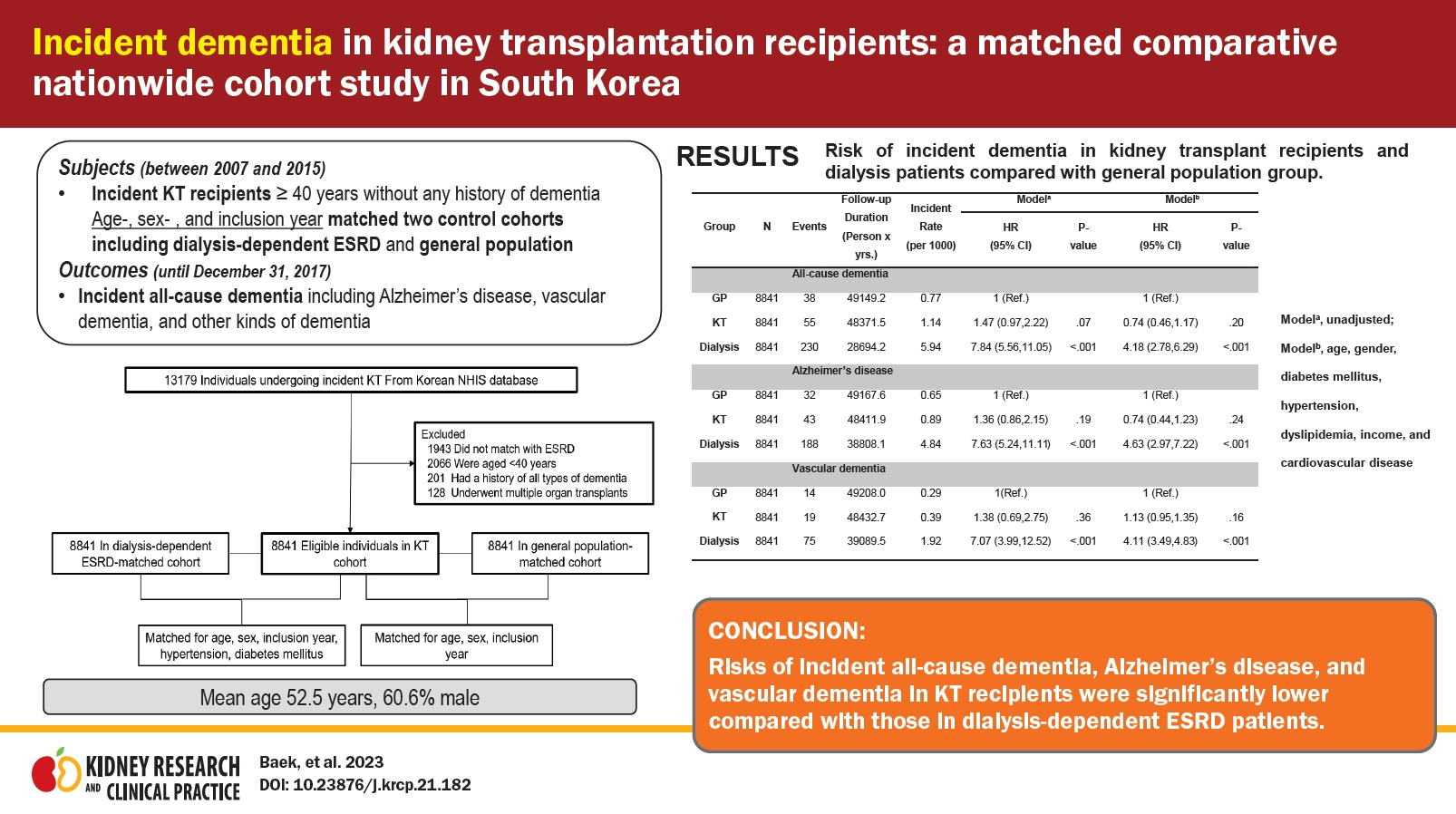
We followed 8,841 KT recipients, dialysis-dependent ESRD patients, and GP individuals for 48,371, 28,649, and 49,149 patient-years, respectively. Their mean age was 52.5 years, and 60.6% were male. Over the observation period, 55/43/19 KT recipients, 230/188/75 dialysis-dependent ESRD patients, and 38/32/14 GP individuals developed all-cause dementia/AD/VD. The risks of incident all-cause dementia, AD, and VD in KT recipients were similar to those in GP (hazard ratio: 0.74 [p = 0.20], 0.74 [p = 0.24], and 0.59 [p = 0.18], respectively) and significantly lower than those in dialysis-dependent ESRD patients (hazard ratio: 0.17 [p < 0.001], 0.16 [p < 0.001], and 0.16 [p < 0.001], respectively). Older age and diabetes mellitus at the time of KT were risk factors for incident all-cause dementia and AD in KT recipients.
Conclusion
This is the first study to show a beneficial impact of KT on incident dementia compared to dialysis dependency.
Introduction
Dementia, a state of persistent and progressive cognitive dysfunction characterized by impairments in memory resulting in a loss of independent function, is a common and destructive geriatric syndrome that affected an estimated 47 million people in 2015 globally [1–4]. Currently, it has an estimated prevalence of 5% to 7% in individuals aged ≥60 years. Given that the number of affected individuals is predicted to be 131 million worldwide by 2050 [2,3], dementia is considered one of the greatest socioeconomic burdens. Aging is an important but non-modifiable risk factor for all-cause dementia. Potentially modifiable risk factors include hypertension, diabetes mellitus, dyslipidemia, cardiovascular disease, cerebrovascular disease, diet, lifestyle, and education [2,3].
Individuals with chronic kidney disease (CKD) are at higher risk for cognitive decline than the general population (GP) due to having both more traditional cardiovascular risk factors (see above) and nontraditional kidney disease-related factors such as albuminuria, retention of uremic metabolites, anemia, polypharmacy, and dialysis-related factors [4,5]. The prevalence of cognitive impairment in patients with CKD ranges from 10% to 40% and is significantly increased in patients with dialysis-dependent end-stage renal disease (ESRD) [4,6]. Cognitive impairment including dementia is likely to display higher rates of nonadherence with treatment regimens and contributes to adverse outcomes including higher risks of death, dialysis withdrawal, and hospitalization among dialysis-dependent ESRD patients [4,6]. Given that there is no specific treatment for dementia, it is encouraging that there appears to be an improvement in cognitive function after kidney transplantation (KT) among vulnerable dialysis-dependent ESRD patients. Improving cognitive function after KT may be attributable to vanishing uremia-related neurotoxicity and reducing CKD-associated comorbidities like cerebrovascular disease [7–10].
KT is considered the best treatment option for ESRD patients mainly due to deriving survival benefits and a better quality of life even with increases in the age and comorbidities of contemporary transplant recipients [11–14]. However, given that KT recipients live longer with a functioning graft compared to dialysis-dependent ESRD patients and take possible neurotoxic immunosuppressants, they might be at risk for incident age-related conditions, including neurodegenerative diseases [15,16]. There are limited data on whether KT lowers the risk of dementia development or not among dialysis-dependent ESRD patients. Therefore, we aimed to estimate the risk of incident dementia in KT recipients compared to dialysis-dependent ESRD patients and the GP using nationwide cohort data in this study.
Methods
This study was conducted according to the 2013 Declaration of Helsinki and good clinical practice guidelines and the study protocol was approved by the Institutional Review Board of Seoul National University Hospital (No. H1903-097-1018). The South Korean government approved access to the Korean National Health Insurance System (NHIS) database for the present study. The need for informed consent was waived because this study retrospectively used data from the NHIS.
Data source
This study was a nationwide population-based cohort study that used information from the Korean National Health Information Database provided by the NHIS. In South Korea, the National Health Security System is a mandatory social insurance program composed of the National Health Insurance and Medical Aid under the supervision of the Ministry of Health and Welfare [17,18]. The NHIS is the only insurer managed by the government, covers ~97% of the Korean population, and provides universal health coverage. Hence, the Korean NHIS retains an extensive database on patient demographics, medical service use, and disease diagnoses according to International Classification of Disease, 10th revision (ICD-10), codes, and lifestyle details that researchers may access. In the claims database, ESRD patients undergoing dialysis or transplantation receive additional insurance coverage, which is also identified by specific insurance codes mandatorily applied for these patients.
Study participants
We analyzed incident KT recipients registered in the Korean National Health Information Database between 2007 and 2015. In addition, we established a pair of control groups composed of dialysis-dependent ESRD patients and GP individuals, respectively, through 1:1 direct matching with the study population. GP individuals without a previous ESRD history were directly matched according to age, sex, and inclusion year, while dialysis-dependent ESRD patients without a previous KT history were directly matched according to age, sex, inclusion year, hypertension, and diabetes mellitus. During matching, those who failed to enter a matched pair were excluded from the study. Subsequently, we excluded matched pairs aged <40 years, those with a history of dementia, or those with prior multi-organ transplantation.
Study outcomes and follow-up
The primary outcome was incident all-cause dementia, including Alzheimer disease, vascular dementia, and other kinds of dementia. Dementia was diagnosed on the basis of relevant ICD-10 codes (F00 or G30 for Alzheimer disease; F01 for vascular dementia; and F02, F03, or G31 for other dementias) with simultaneous prescription of ≥1 medications for dementia (rivastigmine, galantamine, memantine, or donepezil). When there were codes for both Alzheimer disease and vascular dementia, the principal diagnosis was extracted as the final diagnosis. Follow-up assessments were performed from baseline to outcome events, with data censored at death or December 31, 2017. Additional censoring was conducted for each study group under the following conditions: when KT recipients returned to maintenance dialysis, dialysis patients underwent KT, or GP individuals received any renal replacement therapy.
Data collection
We collected demographic characteristics, including age, sex, and economic status. Economic status was divided into a medical aid group or quartile groups based on the individuals’ insurance fees. Baseline comorbidities, including diabetes mellitus (E11–14), hypertension (I10–13 and I15), and dyslipidemia (E78), were identified based on combinations of the medical history, ICD-10 codes, and prescription codes within 3 years before the inclusion date. Baseline cardiovascular diseases (including stable and unstable angina [I20], myocardial infarction [I21–23], ischemic heart disease [I24, I25], heart failure [I50], and cerebrovascular disease [I63–66]) were identified based on ICD-10 codes within 3 years before the inclusion date. Stroke included ischemic stroke (I63, I64) and intracerebral hemorrhage (I60–62), based on the ICD-10 code. Unique codes given for maintenance dialysis (V001 or O7011–7020 for hemodialysis, V003 or O7071–7075 for peritoneal dialysis) and V005 or R3280 for transplantation allowed identification of information regarding kidney replacement therapy [19]. We recorded the duration of dialysis before the inclusion date for KT recipients and dialysis-dependent ESRD patients as continuous variables. We assessed the main dialysis modality as that performed for the longest duration before the inclusion date. Further, if peritoneal dialysis and hemodialysis were performed during similar periods, we recorded the dialysis type as mixed. For the KT recipients, pre-emptive transplantation was defined as having a previous dialysis duration of <3 months. Immunosuppressive agent usage was reported as follows for the KT group: desensitization therapy, including rituximab or plasmapheresis; induction treatment (interleukin-2 receptor antagonist, antithymocyte globulin, or none); and initial maintenance immunosuppressants except for steroids (tacrolimus, cyclosporine, mycophenolate mofetil, or others). No missing values for any variables were present.
Statistical analysis
We described baseline characteristics using means ± standard deviations for continuous variables and as frequencies (percentages) for categorical variables. Among-group differences in continuous and categorical variables were analyzed using the Mann-Whitney U test or Kruskal-Wallis test and the chi-square or Fisher exact test, respectively. We plotted Kaplan-Meier curves to present the cumulative incidence probability of all-cause dementia, Alzheimer disease, and vascular dementia and performed the log-rank test to examine among-group differences. The incidence rate of dementia was calculated as the number of events per 1,000 person-years. Cox proportional hazards analyses were performed to calculate hazard ratios (HRs) for the occurrence of all-cause dementia, Alzheimer disease, and vascular dementia separately in KT recipients compared to those in dialysis-dependent ESRD patients and the GP. A two-sided p-value of <0.05 was considered statistically significant. All analyses and calculations were performed using SAS version 9.4 (SAS Institute).
Results
Baseline characteristics of study participants
After exclusion (Fig. 1), we followed 8,841 KT recipients, dialysis-dependent ESRD patients, and individuals in the GP for 48,371, 28,649, and 49,149 patient-years, respectively, until an outcome event, censoring at death, or December 31, 2017. Table 1 presents among-group comparisons of patients’ baseline characteristics. In the total population, the mean age was 52.5 ± 7.4 years, and 60.6% were male. The proportion of KT recipients with Medical Aid coverage was lower and higher compared to dialysis-dependent ESRD patients and GP individuals, respectively. The proportion of comorbidities such as diabetes mellitus, hypertension, and dyslipidemia in KT recipients was similar and higher compared to those in dialysis-dependent ESRD patients and the GP, respectively. KT recipients received preemptive KT in 32.1% of cases and received hemodialysis mainly before KT. In terms of immunosuppressants, 16.6%, 86.5%, and 82.2% of KT recipients underwent pre-KT desensitization therapy, induction treatment using basiliximab, and immunosuppression maintenance using tacrolimus, respectively.

Study population.
ESRD, end-stage renal disease; KT, kidney transplantation; NHIS, National Health Insurance System.
Between-group comparison of the incidence probability of dementia
Kaplan-Meier curves of the incidence probability of all-cause dementia disease/Alzheimer disease/vascular dementia for up to 10 years according to the three groups are shown in Fig. 2. Within the observation periods, 55, 230, and 38 KT recipients, dialysis-dependent ESRD patients, and GP individuals developed all-cause dementia, respectively. The annual incidence rates of all-cause dementia in KT recipients, dialysis-dependent ESRD patients, and the GP were 1.14, 5.94, and 0.77 per 1,000 person-years, respectively. After adjusting for age, sex, diabetes mellitus, hypertension, dyslipidemia, income, dialysis vintage period, and cardiovascular disease (model 3), KT recipients showed a similar risk of incident all-cause dementia to that of the GP group (adjusted HR, 0.74; 95% confidence interval [CI], 0.46–1.17; p = 0.20) but a significantly lower risk compared to dialysis-dependent ESRD patients (adjusted HR, 0.17; 95% CI, 0.12–0.23; p < 0.001). During the follow-up period, Alzheimer disease and vascular dementia were found in 43/19 KT recipients, 188/75 dialysis-dependent ESRD patients, and 32/14 GP individuals, respectively. Similar findings were noted for the risk of incident Alzheimer disease and vascular dementia (Alzheimer disease: adjusted HR, 0.74 [95% CI, 0.44–1.23] in KT recipients vs. the GP group and adjusted HR, 0.16 [95% CI, 0.11–0.22] in KT recipients vs. dialysis-dependent ESRD patients; vascular dementia: adjusted HR, 0.59 [95% CI, 0.27–1.28] in KT recipients vs. the GP group and adjusted HR, 0.16 [95% CI, 0.10–0.27] in KT recipients vs. dialysis-dependent ESRD patients) (Table 2, 3).
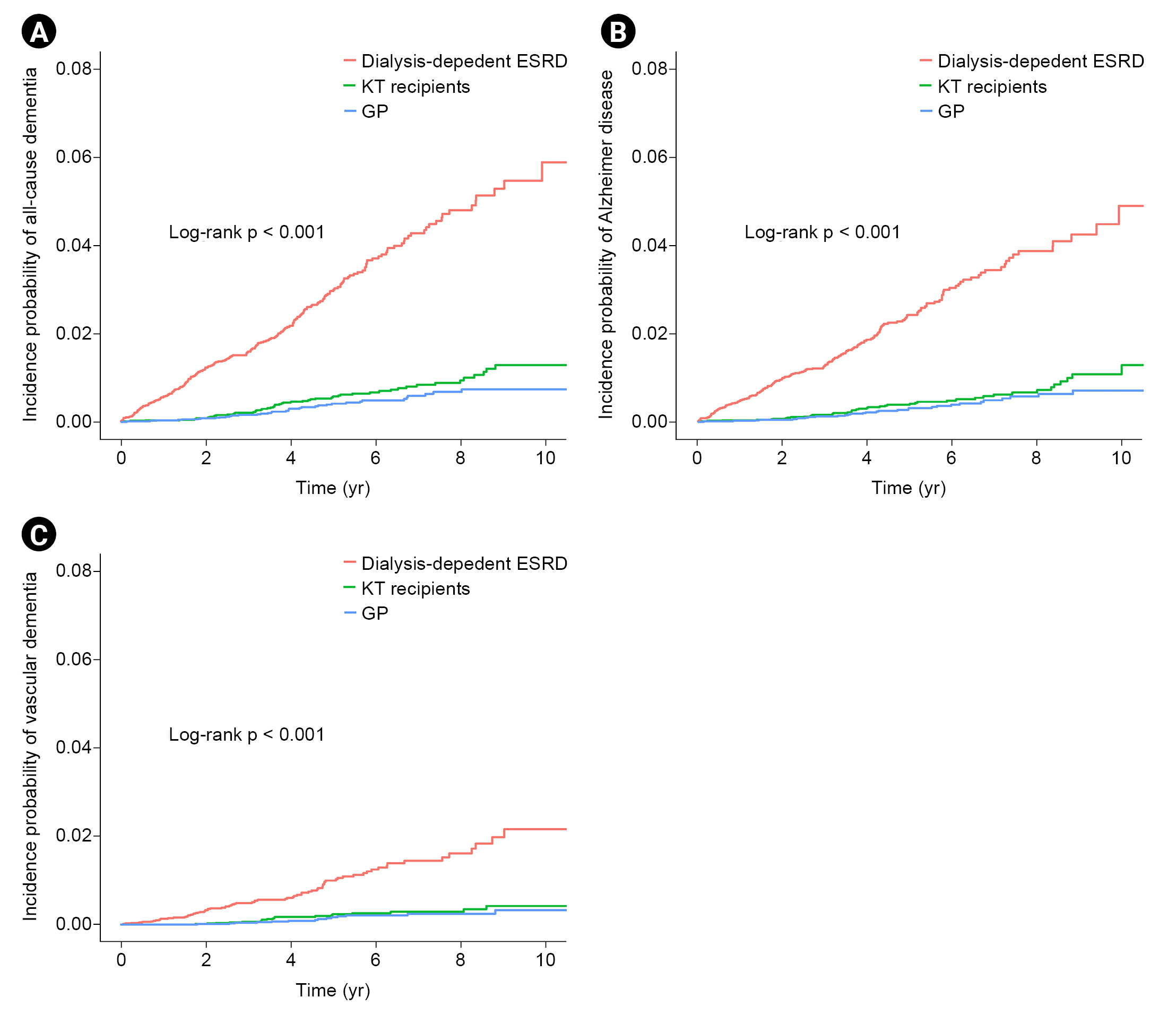
Comparison of disease risk among KT, dialysis-dependent ESRD, and GP groups.
(A) Risk of all-cause dementia. (B) Risk of Alzheimer disease. (C) Risk of vascular dementia.
ESRD, end-stage renal disease; GP, general population; KT, kidney transplantation.
Predictors of dementia in kidney transplantation recipients
The recipients with incident all-cause dementia were older at the time of KT (52.4 years vs. 60.5 years, p < 0.001) and had higher proportions of diabetes mellitus (42.7% vs. 69.1%, p < 0.001), dyslipidemia (56.1% vs. 70.9, p = 0.04), and cardiovascular disease (24.2% vs. 38.2%, p = 0.03) at the time of transplantation compared to KT recipients without dementia (Supplementary Table 1, available online). In the multivariable Cox regression analysis, the risk of all-cause dementia increased with age at KT. Additionally, recipients with diabetes mellitus were at increased risk of developing all-cause dementia (adjusted HR, 2.01 [95% CI, 1.13–3.79]; p = 0.02). However, incident all-cause dementia was not associated with hypertension, dyslipidemia, or cardiovascular disease. Moreover, there were no associations of incident all-cause dementia with sex, income, dialysis vintage period, and immunosuppressants (Table 4). Similarly, the recipients with incident Alzheimer disease were older at the time of KT and had a higher proportion of diabetes mellitus compared to recipients without Alzheimer disease (Supplementary Table 2, available online). Similarly, KT recipients with incident vascular dementia were older than those without vascular dementia (Supplementary Table 3, available online). In the multivariable Cox regression analysis, older age and diabetes mellitus at the time of KT were risk factors for Alzheimer disease (Supplementary Table 4, available online). Diabetes mellitus was also a risk factor for vascular dementia (Supplementary Table 5, available online).
Discussion
In this nationwide population-based longitudinal cohort study, we evaluated the risks of incident all-cause dementia, Alzheimer disease, and vascular dementia in KT recipients compared to those in dialysis-dependent ESRD patients and the GP, respectively. We identified that the risks of incident all-cause dementia, Alzheimer disease, and vascular dementia in KT recipients were significantly lower and similar compared to both those in dialysis-dependent ESRD patients and the GP. Older age and diabetes mellitus at the time of KT were risk factors for incident all-cause dementia and Alzheimer disease in KT recipients, and diabetes mellitus at the time of KT was also a risk factor for incident vascular dementia in KT recipients. To our knowledge, this is the first study to show the beneficial effect of KT on de novo dementia in ESRD patients compared to dialysis-dependent patients. Interestingly, from this study, we determined that the risk of incident dementia in KT recipients was comparable even to that of the GP when matched according to age and sex.
An elevated risk of cognitive impairment, including incident dementia, in dialysis patients has been explored in previous studies [4,5,20–22]. Moreover, dialysis-dependent ESRD itself is considered an etiological risk factor for dementia according to a cause-specific hazard model [23]. Consistent with previous studies, our results revealed that dialysis-dependent ESRD patients have an increased risk of incident all-cause dementia/Alzheimer disease/vascular dementia compared to the GP. Pathophysiologic mechanisms for the occurrence and progression of dementia in patients with CKD and dialysis-dependent ESRD have been reported to be traditional risk factors like older age, hypertension, diabetes mellitus, dyslipidemia, and cardiovascular disease; nontraditional kidney disease-related factors such as albuminuria, retention of uremic toxin, anemia, and vascular calcification; and ESRD treatment-associated risk factors including intradialytic hypotension, cerebral edema, and thrombotic events [4,6,20,24–32].
More recently, KT recipients have been reported to have a greater risk for post-KT dementia than previously published estimates of the dementia risk among GP individuals [33]. However, the risks of incident all-cause dementia and specific subtypes of dementia in KT recipients are similar to those in the GP according to the results of our study. A simple comparison of the incidence between KT recipients and the GP without adjustment of comorbidities in different individual cohorts was postulated as the cause for the elevated risk of dementia. In our data, the dementia risk of KT recipients was not higher than that of the GP after adjustment of demographics, comorbidities, and income. A previously published epidemiological study reported that the incidence of age- and sex-standardized dementia was 8.6/1,000 person-years in those with >65 years of age [34]. In the present study, the incidence of dementia was 0.8/1,000 person-years in the GP with >40 years of age—a finding that appears low because subjects aged 40 to 65 years were included in the study. Because most dementia-related epidemiologic studies have been conducted in population groups with >65 years of age [35], we reported that the incidence of dementia in patients aged ≥65 years was 5.8/1,000 person-years in our cohort—a finding that is similar to previous results (Supplementary Table 6, available online). Consistent with the results of a previously published study [33], older age and diabetes mellitus at the time of KT are risk factors for incident all-cause dementia and Alzheimer disease according to the present study.
We found that KT recipients have a significantly lower risk of incident all-cause dementia/Alzheimer disease/vascular dementia than dialysis-dependent ESRD patients. Why is KT related to a lower risk of dementia than that of dialysis-dependent ESRD patients? Pathophysiologically, KT may reverse the underlying mechanisms that cause cognitive impairment in ESRD patients, including nontraditional kidney disease-related factors and ESRD treatment-associated factors, as mentioned above [15]. A previous study reported that high middle molecule clearance rates improve general cognitive and executive functions in patients undergoing peritoneal dialysis [31]. Since insufficient middle molecule clearance (a limitation of current dialysis) is improved in post-KT patients, it may have an impact on the low incidence of dementia found. Second, protein-bound uremic toxin is associated with cognitive function in hemodialysis patients [32]. Protein-bound uremic toxin removal is also a limitation of current dialysis and may explain the low incidence of dementia found in post-KT patients. Third, the risk of incident stroke in KT recipients was significantly lower and similar to those in dialysis-dependent ESRD patients and the GP, respectively, based on the results of our study (Supplementary Table 7, available online). These results may explain why vascular dementia was lower in the KT group than in dialysis-dependent ESRD patients. Several studies have addressed the overall beneficial effect of transplantation on cognitive function [4,6–8,10,15,36,37]. However, these studies enrolled small sample sizes and focused on neuropsychological performance, not dementia. To our knowledge, this is the first large-scale cohort study to estimate the risks of incident all-cause dementia and dementia subtypes among KT recipients compared to dialysis-dependent ESRD patients. Furthermore, given that there are limited data on the efficacy or safety of pharmacological therapy for dementia in ESRD patients [4] and dialysis may not offer the same benefits as having normal kidney function [15], reducing the risk of dementia may be a reason for recommending KT in dialysis-dependent ESRD patients at high risk of incident dementia.
This study has several limitations. First, we did not use dialysis-dependent ESRD patients awaiting KT as a matched control group because we did not have information on the transplant waitlist. Therefore, potential selection bias remains. Second, due to the limited information obtainable from claims data, we could not adjust for several variables that can affect the occurrence of dementia, including lifestyle factors like exercise and sleep habits; laboratory findings such as hemoglobin, parathyroid hormone, and uremic toxin concentrations; and dialysis adequacy. Third, we analyzed observation data; therefore, it remains unclear why KT recipients are less likely to develop dementia. Nonetheless, this is the first large-scale study to compare the incidence of all-cause dementia, Alzheimer disease, and vascular dementia among KT recipients, dialysis-dependent ESRD patients, and the GP using a nationwide dataset in which the insured are covered by single compulsory insurance with a 100% claims rate. Our findings demonstrated that KT is associated with a lower risk of dementia compared to that of dialysis-dependent ESRD patients. Therefore, KT could be suggested for dialysis-dependent ESRD patients at risk of incident dementia.
In conclusion, this epidemiologic study provides evidence that the risks of incident all-cause dementia, Alzheimer disease, and vascular dementia in KT recipients are significantly lower than those of dialysis-dependent ESRD patients and similar to those in the GP even after adjustment for potential confounding factors. Moreover, we found that older age and diabetes mellitus at the time of KT are independent risk factors for the onset of all-cause dementia and Alzheimer disease in KT recipients. Diabetes mellitus at the time of KT is also a risk factor for the development of vascular dementia in KT recipients.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Funding
This research was supported in study design and data collection by the grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HI18C1604).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
Authors’ contributions
Conceptualization, Investigation, Methodology: SHB, SP, MY, JEK, KH, YCK, HL
Formal analysis: JP, SP, KH, SHP
Funding acquisition: HL
Resources: DKK, KWJ, YSK
Writing–original draft: SHB, HL
Writing–review & editing: All authors
All authors read and approved the final manuscript.
Supplementary Materials
Supplementary data are available at Kidney Research and Clinical Practice online (https://doi.org/10.23876/j.krcp.21.182).