| Kidney Res Clin Pract > Volume 42(4); 2023 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This research was supported in study design and data collection by the grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No. HI18C1604).
Data sharing statement
The data presented in this study are available on request from the corresponding author.
AuthorsŌĆÖ contributions
Conceptualization, Investigation, Methodology: SHB, SP, MY, JEK, KH, YCK, HL
Formal analysis: JP, SP, KH, SHP
Funding acquisition: HL
Resources: DKK, KWJ, YSK
WritingŌĆōoriginal draft: SHB, HL
WritingŌĆōreview & editing: All authors
All authors read and approved the final manuscript.
Supplementary Materials
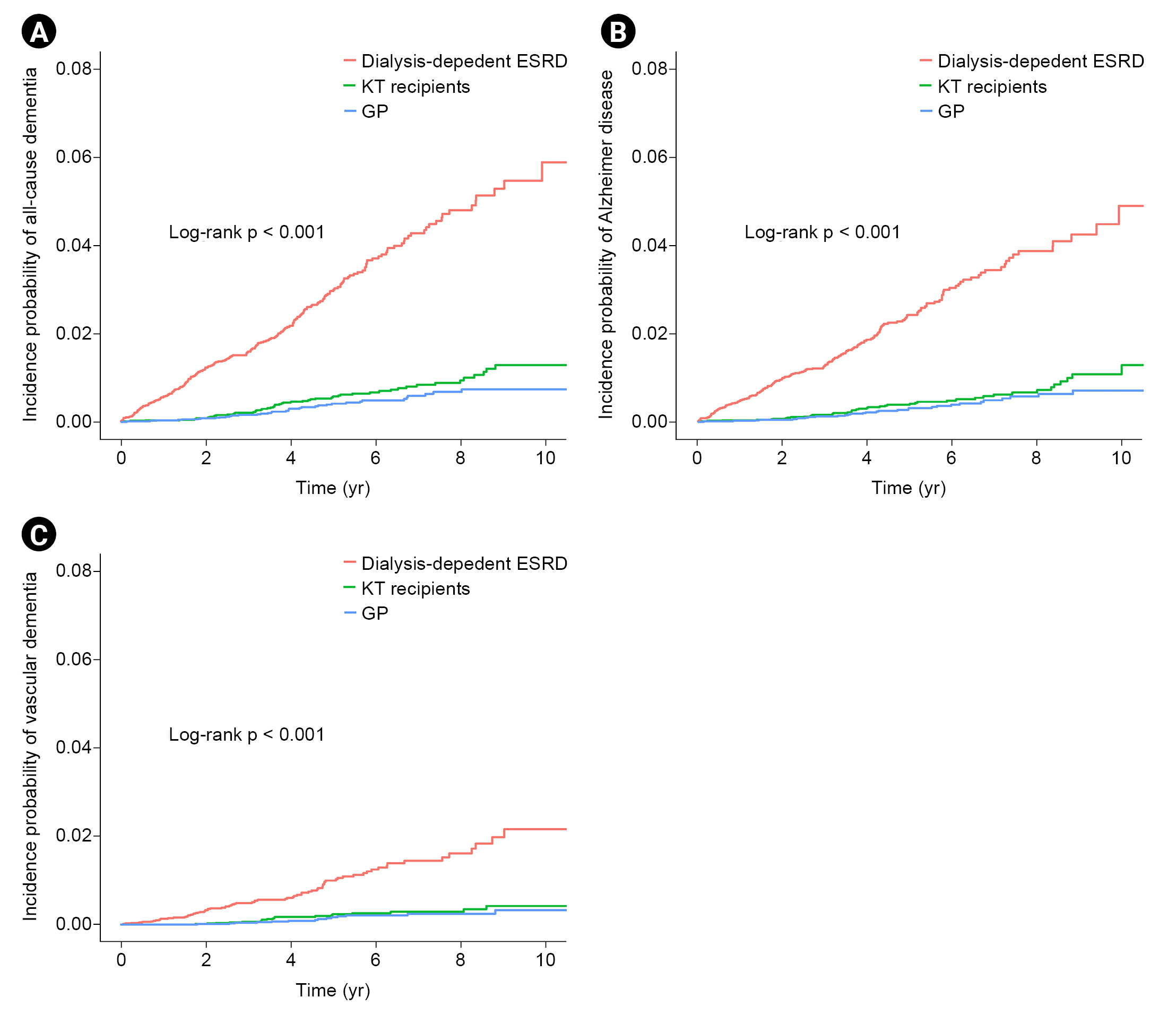
Figure┬Ā1.
Study population.

Figure┬Ā2.
Comparison of disease risk among KT, dialysis-dependent ESRD, and GP groups.
Table┬Ā1.
Table┬Ā2.
| Group | Number | Events | Follow-up (person-yr) | Incident rate (per 1,000) |
Modela |
Modelb |
Modelc |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |||||
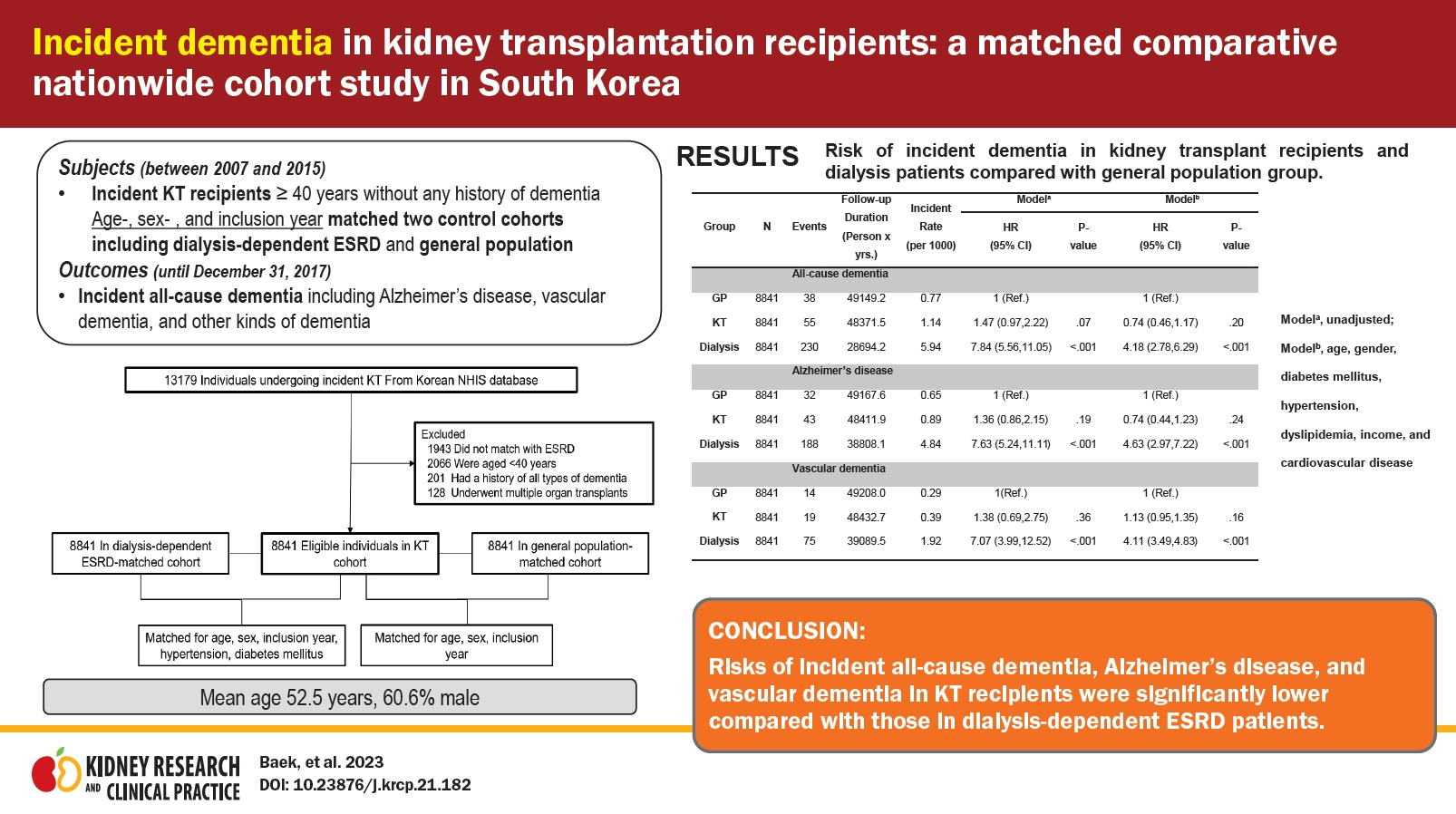
| All-cause dementia | ||||||||||
| ŌĆāGP | 8,841 | 38 | 49,149.2 | 0.77 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ŌĆāKT | 8,841 | 55 | 48,371.5 | 1.14 | 1.47 (0.97ŌĆō2.22) | 0.07 | 1.52 (1.00ŌĆō2.29) | 0.05 | 0.74 (0.46ŌĆō1.17) | 0.20 |
| ŌĆāDialysis | 8,841 | 230 | 28,694.2 | 5.94 | 7.84 (5.56ŌĆō11.05) | <0.001 | 8.62 (6.11ŌĆō12.17) | <0.001 | 4.18 (2.78ŌĆō6.29) | <0.001 |
| Alzheimer disease | ||||||||||
| ŌĆāGP | 8,841 | 32 | 49,167.6 | 0.65 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ŌĆāKT | 8,841 | 43 | 48,411.9 | 0.89 | 1.36 (0.86ŌĆō2.15) | 0.19 | 1.42 (0.90ŌĆō2.24) | 0.14 | 0.74 (0.44ŌĆō1.23) | 0.24 |
| ŌĆāDialysis | 8,841 | 188 | 38,808.1 | 4.84 | 7.63 (5.24ŌĆō11.11) | <0.001 | 8.57 (5.88ŌĆō12.50) | <0.001 | 4.63 (2.97ŌĆō7.22) | <0.001 |
| Vascular dementia | ||||||||||
| ŌĆāGP | 8,841 | 14 | 49,208 | 0.29 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ŌĆāKT | 8,841 | 19 | 48,432.7 | 0.39 | 1.38 (0.69ŌĆō2.75) | 0.36 | 1.42 (0.71ŌĆō2.83) | 0.32 | 1.13 (0.95ŌĆō1.35) | 0.16 |
| ŌĆāDialysis | 8,841 | 75 | 39,089.5 | 1.92 | 7.07 (3.99ŌĆō12.52) | <0.001 | 7.61 (4.30ŌĆō13.49) | <0.001 | 4.11 (3.49ŌĆō4.83) | <0.001 |
Table┬Ā3.
| Group | Number | Events | Follow-up (person-yr) | Incident rate (per 1,000) |
Modela |
Modelb |
Modelc |
|||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | HR (95% CI) | p-value | |||||
| All-cause dementia | ||||||||||
| ŌĆāDialysis | 8,841 | 230 | 28,694.2 | 5.94 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ŌĆāKT | 8,841 | 55 | 48,371.5 | 1.14 | 0.19 (0.14ŌĆō0.25) | <0.001 | 0.18 (0.13ŌĆō0.24) | <0.001 | 0.17 (0.12ŌĆō0.23) | <0.001 |
| Alzheimer disease | ||||||||||
| ŌĆāDialysis | 8,841 | 188 | 38,808.1 | 4.84 | 1 (Reference) | 1 (Reference) | <0.001 | 1 (Reference) | ||
| ŌĆāKT | 8,841 | 43 | 48,411.9 | 0.89 | 0.18 (0.13ŌĆō0.25) | <0.001 | 0.17 (0.12ŌĆō0.23) | <0.001 | 0.16 (0.11ŌĆō0.22) | <0.001 |
| Vascular dementia | ||||||||||
| ŌĆāDialysis | 8,841 | 75 | 39,089.5 | 1.92 | 1 (Reference) | 1 (Reference) | 1 (Reference) | |||
| ŌĆāKT | 8,841 | 19 | 48,432.7 | 0.39 | 0.20 (0.12ŌĆō0.33) | <0.001 | 0.19 (0.11ŌĆō0.31) | <0.001 | 0.16 (0.10ŌĆō0.27) | <0.001 |
Table┬Ā4.
| Variable |
Modela |
Modelb |
||
|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |
| Age (yr) | ||||
| ŌĆā40ŌĆō44 | 0 (0) | >0.99 | 0 (0) | >0.99 |
| ŌĆā45ŌĆō49 | 0.47 (0.12ŌĆō1.825) | 0.28 | 0.49 (0.13ŌĆō1.91) | 0.31 |
| ŌĆā50ŌĆō54 | 1 (Reference) | 1 (Reference) | ||
| ŌĆā55ŌĆō59 | 3.02 (1.22ŌĆō7.48) | 0.02 | 2.79 (1.12ŌĆō6.94) | 0.03 |
| ŌĆā60ŌĆō64 | 5.00 (2.01ŌĆō12.35) | 0.001 | 4.54 (1.81ŌĆō11.38) | 0.001 |
| ŌĆā65ŌĆō69 | 13.71 (5.52ŌĆō34.03) | <0.001 | 12.90 (5.09ŌĆō32.70) | <0.001 |
| ŌĆāŌēź70 | 17.09 (4.39ŌĆō66.60) | <0.001 | 13.44 (3.38ŌĆō53.35) | <0.001 |
| Sex | ||||
| ŌĆāFemale vs. male | 0.83 (0.48ŌĆō1.44) | 0.50 | 1.03 (0.58-1.82) | 0.93 |
| Income | ||||
| ŌĆāMedical aid | 1 (Reference) | 1 (Reference) | ||
| ŌĆāQ1 | 1.41 (0.43ŌĆō4.62) | 0.57 | 0.65 (0.18ŌĆō2.03) | 0.42 |
| ŌĆāQ2 | 2.42 (0.84ŌĆō6.97) | 0.10 | 0.92 (0.31ŌĆō2.73) | 0.88 |
| ŌĆāQ3 | 1.24 (0.41ŌĆō3.80) | 0.70 | 0.46 (0.15ŌĆō1.43) | 0.18 |
| ŌĆāQ4 | 2.10 (0.80ŌĆō5.49) | 0.13 | 0.72 (0.26ŌĆō1.95) | 0.51 |
| Diabetes mellitus | 3.38 (1.90ŌĆō5.99) | <0.001 | 2.07 (1.13ŌĆō3.79) | 0.02 |
| Hypertension | 4.57 (0.63ŌĆō33.07) | 0.13 | 2.87 (0.39ŌĆō21.13) | 0.30 |
| Dyslipidemia | 2.30 (1.29ŌĆō4.13) | 0.005 | 1.53 (0.84ŌĆō2.81) | 0.17 |
| Cardiovascular disease | 2.12 (1.23ŌĆō3.65) | 0.007 | 1.18 (0.67ŌĆō2.07) | 0.57 |
| Dialysis vintage period (yr) | 1.00 (0.91ŌĆō1.09) | 0.97 | 1.02 (0.93ŌĆō1.12) | 0.68 |
| Immunosuppressants | ||||
| ŌĆāDesensitization | 1.64 (0.84ŌĆō3.21) | 0.14 | 1.59 (0.80ŌĆō3.17) | 0.19 |
| ŌĆāInduction | ||||
| ŌĆāŌĆāATG | 1 (Reference) | 0.91 | 1 (Reference) | 0.86 |
| ŌĆāŌĆāBasiliximab | 0.94 (0.34ŌĆō2.61) | 1.10 (0.39ŌĆō3.12) | ||
| ŌĆāMaintenance CNI | ||||
| ŌĆāŌĆāTacrolimus | 1 (Reference) | 0.77 | 1 (Reference) | 0.82 |
| ŌĆāŌĆāCyclosporine | 0.90 (0.45ŌĆō1.80) | 1.08 (0.54ŌĆō2.18) | ||
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,496 View
- 86 Download
- ORCID iDs
-
Seon Ha Baek

https://orcid.org/0000-0002-4751-9817Jina Park

https://orcid.org/0000-0002-7410-1985Sehoon Park

https://orcid.org/0000-0002-4221-2453Mi-yeon Yu

https://orcid.org/0000-0001-5112-6955Ji Eun Kim

https://orcid.org/0000-0003-3094-2229Sang Hyun Park

https://orcid.org/0000-0003-0612-2562Kyungdo Han

https://orcid.org/0000-0002-6096-1263Yong Chul Kim

https://orcid.org/0000-0003-3215-8681Dong Ki Kim

https://orcid.org/0000-0002-5195-7852Kwon Wook Joo

https://orcid.org/0000-0001-9941-7858Yon Su Kim

https://orcid.org/0000-0003-3091-2388Hajeong Lee

https://orcid.org/0000-0002-1873-1587 - Related articles
-
Impact of chronic kidney disease on mortality: A nationwide cohort study2019 September;38(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















