Healthy aging and chronic kidney disease
Article information
Abstract
The world population is aging and the prevalence of noncommunicable diseases such as chronic kidney disease (CKD) will increase significantly. With advances in medical treatment and public health, the human lifespan continues to outpace the health span and the last decade of life is generally spent in poor health. In 2015, the World Health Organization defined healthy aging as ‘the process of developing and maintaining the functional ability that enables wellbeing in older age.’ CKD is increasingly being recognized as a model of accelerated aging and is associated with physical performance decline, cognitive decline, falls and fractures, poor quality of life, loss of appetite, and inflammation. Frailty and dementia are the final pathways and key determinants of disability and mortality independent of underlying disease. CKD, dementia, and frailty share a triangular relationship with synergistic actions and have common risk factors wherein CKD accelerates frailty and dementia through mechanisms such as uremic toxicity, metabolic acidosis and derangements, anorexia and malnutrition, dialysis-related hemodynamic instability, and sleep disturbance. Frailty accelerates glomerular filtration decline as well as dialysis induction in CKD and more than doubles the mortality risk. Anorexia is one of the major causes of protein-energy malnutrition, which is also prevalent in the aging population and warrants screening. Healthcare systems across the world need to have a system in place for the prevention of CKD amongst high-risk older adults, focusing on screening for poor prognostic factors amongst patients with CKD such as frailty, poor appetite, and cognitive impairment and providing necessary person-centered interventions to reverse underlying factors that may contribute to poor outcomes.
Introduction
The world population is aging at an exponential rate and demographic transition will have a major impact on health and social care costs. The lifespan continues to outpace healthspan, resulting in older adults spending their last decade in poor health. In the aging population, the prevalence of noncommunicable diseases such as chronic kidney disease (CKD), cardiovascular disease, diabetes, hyperlipidemia, and neurodegenerative diseases with associated frailty and dementia is expected to increase significantly. By 2040, CKD is projected to be the fifth leading cause of death across the world [1]. The world report on aging and health published by the World Health Organization (WHO) in 2015 defined ‘healthy aging’ as ‘the process of developing and maintaining the functional ability that enables wellbeing in older age’ with a particular focus on intrinsic capacities such as sensory impairment, cognition, nutrition, mobility, and depression [2,3]. The trajectory of aging is determined by multiple complex processes, including genetic susceptibility as well lifestyle, chronic diseases, behavioral, environmental, and dietary factors. Frailty and dementia are the main determinants of disability and are associated with increased morbidity and mortality independent of the underlying disease.
The current disease-centric and acute reactive healthcare model is not sustainable in countries with a fast-growing aging population and for conditions like CKD where there is a high prevalence of polypharmacy, multimorbidity, and heterogeneity. The healthcare systems of many countries are moving from a reactive state to a proactive one focusing on preventive care [4]. Redesigning of healthcare care is necessary and the WHO has published guidelines advocating on proactive assessments (e.g., comprehensive geriatric assessment) of individual impairments, slowing the decline in capacity with personalized care plans and providing interventions to improve nutrition and physical exercise and referrals to specialists for those with impairments [3,5].
With aging, the kidney undergoes functional and structural changes. Microscopic changes include glomerulosclerosis, interstitial fibrosis, thickening of the glomerular basement membrane, arteriosclerosis and tubular atrophy with a consequent decline in renal mass, glomerular filtration, and autoregulatory function [6]. In addition to the reduced functional reserve, chronic diseases such as diabetes, nephrotoxic polypharmacy, and sepsis are more prevalent in this group, and—together with underlying physiological changes such as a lack of thirst—can increase susceptibility to acute kidney injury [7]. CKD is considered a model of accelerated aging and is associated with a decline in physical performance, cognitive decline, fatigue, falls and fractures, poor quality of life (QoL), loss of appetite, and inflammation [8]. The term ‘senescent nephropathy’ is used to describe the synergistic decline in renal and physical function and is thought to be caused by increased inflammation in both aging and CKD [9]. While low-grade inflammation is not uncommon in aging, inflammation in CKD can be caused by dialysis-related factors (biocompatible membranes, dialysate [e.g., endotoxins], vascular access [e.g., prosthetic arteriovenous grafts/catheters], uremic toxicity, volume overload, subclinical infections, and alterations in the gut microbiome) [10]. Early identification of frailty and cognitive impairment in older patients with CKD will guide risk stratification and, when accompanied by personalized interventions, may promote aging free from disability as well as better self-rated health.
Frailty, cognitive impairment, and chronic kidney disease
There is a triangular relationship between dementia/cognitive impairment, frailty, and CKD with aging (Fig. 1) [11,12]. The common pathophysiology and risk factors include comorbidities that often coexist in older adults, vascular risk factors such as arteriosclerosis, physical inactivity, inflammation, mitochondrial dysfunction, nutrition, depression, and impairments of the senses such as vision and hearing. Management of extrarenal complications such as frailty and cognitive impairment is a rapidly developing area in nephrology and there is lack of consensus on screening, interventions, and intended outcomes. Frailty is a dynamic, multidimensional state affecting multiple physiological systems that increases vulnerability to stressors, resulting in functional decline, falls, and increased morbidity and mortality rates [13]. The prevalence of frailty and pre-frailty in older adults worldwide varies from 43%–60% to 78% in hemodialysis (HD) patients [14]. It is an independent marker for diabetes and declines in kidney function and cognition [12,15–19]. The prevalence of frailty in pre-dialysis patients is as high as 43% and they are 2.5 times more likely to die or to begin dialysis [9,12]. Frailty may be reversible, especially before the onset of disability, with targeted interventions such as nutrition, exercise, polypharmacy, and oral health management [16,20]. However, despite its poor outcomes, frailty screening has not been incorporated into routine care by nephrologists.
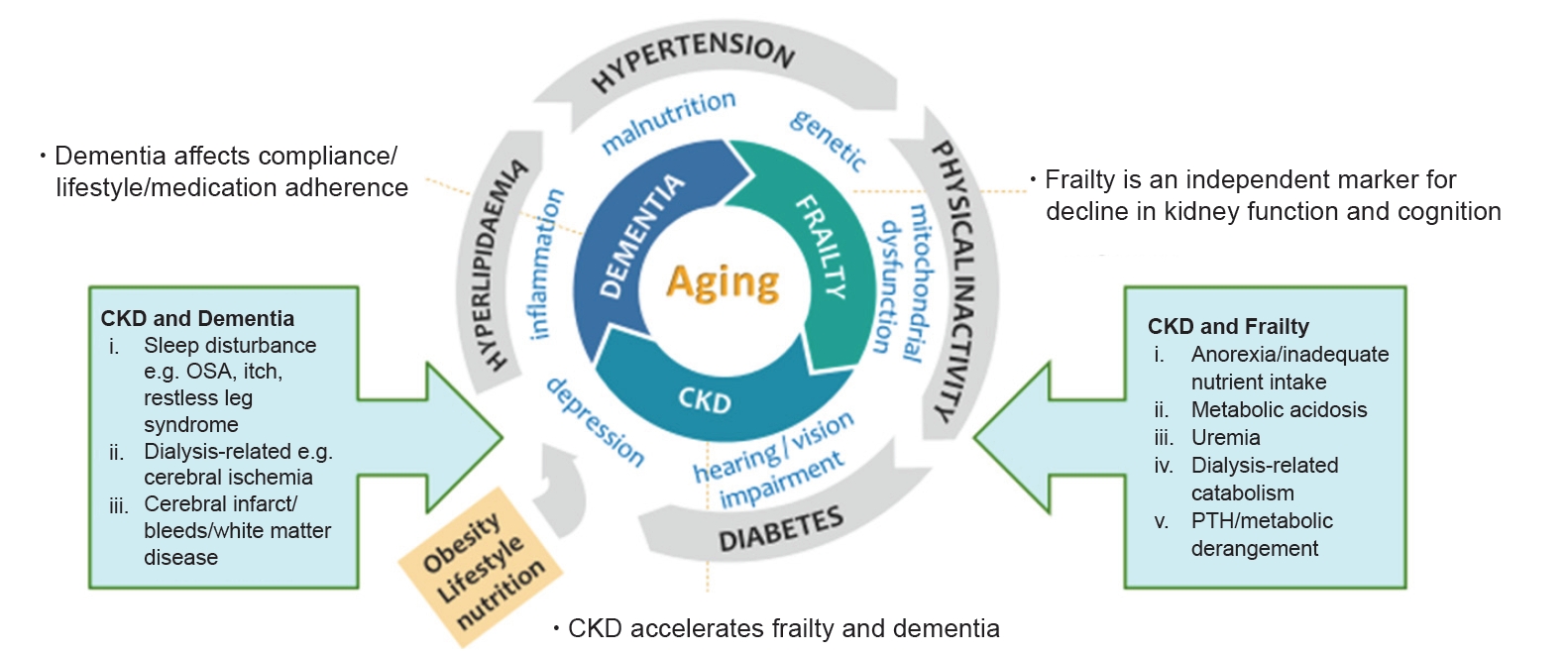
Frailty, CKD, and dementia in the aging population.
Triangular relationship, synergistic actions, and common pathways.
CKD, chronic kidney disease; OSA, obstructive sleep apnoea; PTH, parathyroid hormone.
Albuminuria and CKD serve as complimentary risk factors for cognitive decline. Risk factors for cognitive decline can broadly be divided into three main groups: traditional risk factors applicable to older adults in general; dialysis-related factors; and nontraditional risk factors such as malnutrition, anemia, and inflammation [12,21].
There is a significant symptom burden among patients with CKD, some of which could be attributed to frailty and cognitive impairment, which is not often assessed in a clinical setting. In patients with CKD stage 4 to 5 managed without dialysis, weakness was found in 75%, poor mobility in 75%, poor appetite in 58%, pain in 56%, pruritus in 56%, and dyspnea in 49% [22].
Frailty and chronic kidney disease
A low estimated glomerular filtration rate (eGFR) is associated with a greater risk of frailty, and frail patients with CKD do much worse. In pre-dialysis patients, frailty is associated with a faster decline in eGFR, accelerated dialysis initiation, poor QoL, and increased mortality [9]. In dialysis patients, frailty is associated with increased risk of falls, readmission to the hospital, and more than double odds of mortality in 1 year [9,23]. Decreased energy and food intake with declining eGFR, in addition to other factors like metabolic acidosis, uremia, dialysis-related catabolism, hospital admissions, and metabolic derangement, contributes to sarcopenia and physical frailty (Fig. 1) [13,24]. Sarcopenia is a component of physical frailty defined as a progressive loss of skeletal muscle mass, quality and strength that is highly prevalent in CKD and has recently been shown to be associated with intradialytic hypotension in addition to other adverse outcomes such as mortality [25].
The operational definition of frailty is based on two principal concepts: the Fried Phenotype Model of Physical Frailty and the Cumulative Deficit Model of Frailty. The Fried Phenotype Model of Physical Frailty was validated in the Cardiovascular Health Study and is based on quantification, which requires measurements such as gait speed, handgrip strength, and physical activity in addition to weight loss and exhaustion [26]. The fatigue, resistance, ambulation, illnesses, and loss of weight (FRAIL) scale is based on a similar concept but relies on self-reported questionnaire responses pertaining to items such as fatigue, difficulty walking one block of 50 m, difficulty climbing a flight of stairs, weight loss and ≥5 illnesses [27,28]. The Rockwood Mitnitsky Frailty Index is a 70-item multi-domain frailty phenotype based on self-reported data initially validated in the Canadian study of health and aging that determines deficits in functional, cognitive, and social domains. The number of domains can be simplified and frailty is measured based on a ratio of the number of health deficits present over the number of health deficits measured [29]. The common physical function and frailty assessment tools are listed in Table 1 [3,9,26,27,29–36].
The short physical performance battery test covers three domains: balance, gait speed, and sit-to-stand. The test has been shown to be associated with disease progression and increased mortality [9]. Gait speed alone has been shown to correlate with frailty and predict cognitive impairment, hospitalization, and mortality [30,37]. van Loon et al. [14] compared various frailty screening tools, including the Fried Frailty Phenotype, Groningen Frailty Indicator, Geriatric 8, the Identification of Seniors at Risk and the Hospital Safety Program, where the sensitivity ranged from 48% (Fried Frailty Phenotype) to 88% (Geriatric 8). Anderson et al. [38] compared the Frailty Index, Frailty Phenotype, Edmonton Frail Scale, and Clinical Frailty Scale in HD patients and found that agreement between different frailty tools was weak and the tools themselves were not interchangeable. The selection of an optimal frailty screening tool requires the balance between utility in decision-making, interventions or outcomes intended and ease of administration.
Cognitive impairment and chronic kidney disease
The presence of cognitive impairment in patients with CKD was first described by Dr. Thomas Addison in 1839 and, despite its significant association with poor treatment compliance, decision-making, readmissions, worsening kidney function, falls, and mortality, screening for cognitive impairment is not routinely conducted in the nephrology clinic [39]. More than half of HD patients with cognitive impairment are nonadherent to their medications [40]. It is estimated that every 10 mL/min/1.73 m2 decrease in eGFR is associated with an 11% increase in cognitive impairment prevalence amongst those aged >55 years. There is a rapid decline in cognition for those with eGFR of <30 mL/min/1.73 m2, where more than three quarters of those undergoing dialysis and up to 2/3 of pre-dialysis patients may have underlying cognitive impairment [32,39]. The initiation of dialysis can cause a stepwise decline in cognition, especially executive function [41]. Lacunar infarcts, white matter disease, and cerebrovascular disease are also common in CKD patients, especially those on dialysis, and intradialytic hemodynamic disturbances can precipitate cerebral ischemia with a cumulative impact on cognitive function [42]. Other factors often overlooked include sleep disturbance due to obstructive sleep apnea or restless leg syndrome, depression, anemia, and nutrient deficiencies [43]. While kidney transplantation is the gold standard for improvement in cognition in those with end-stage kidney disease, peritoneal dialysis (PD) also appears to be ‘protective’ in some studies [44].
There is still an ongoing debate about which is the best practical test to assess cognitive impairment in patients with CKD. Similar to frailty, the choice of test is dependent on its utility, time taken, threshold for intervention, and intended outcome overall. Cognitive tests (Table 2) [39,45–52] can vary from neuropsychological assessment, which takes >60 minutes in some patients and is costly to perform, to brief methods of triage, such as a single screening question or the Rapid Cognitive Screen or Mini-Cog exam [45]. Multidomain screening methods, such as the Mini-Mental State Examination and the Montreal Cognitive Assessment (which takes up to 15 minutes) are frequently used in clinical practice [39,46,47]. There are many interventions that can improve or slow down cognitive decline, requiring further validation in larger CKD populations while considering aspects such as cooled dialysis, type of dialysis, renal transplantation, anemia correction, supplements, nutrition, and exercise [39].
Anorexia of aging and chronic kidney disease
Anorexia of aging (AA) is defined as a reduced desire to eat or a loss of appetite in older adults, which is associated with weight loss, frailty, sarcopenia, hospitalization, disability, and mortality. The decrease in appetite may affect food intake and/or type of food intake, affecting overall energy intake and causing a loss of weight, which in turn is a precursor of frailty and loss of muscle mass. AA can be caused by the dysfunctional release of hormones (e.g., cholecystokinin, ghrelin, leptin), which also occurs in CKD, underlying diseases and polypharmacy, a decline in physical or mental health (including depression and dementia), a decrease in fundal compliance, poor oral health, xerostomia, and social factors such as loneliness (Fig. 2) [53,54]. In addition to the above factors, causes of anorexia in patients with CKD with/without dialysis can include a low acyl-ghrelin level, high leptin concentration, proinflammatory cytokines, metabolic acidosis, uremic toxins, changes in gut microbiomes, dialysis-related factors such as fatigue, nausea, vomiting, impaired gastric emptying and bloating, metabolic derangement, and altered taste perception [55–57]. The combination of anorexia and dietary restrictions not personalized to age, function, culture, comorbidities, or goals of treatment in the background of inflammation are major causes of protein-energy malnutrition (PEM) in CKD.
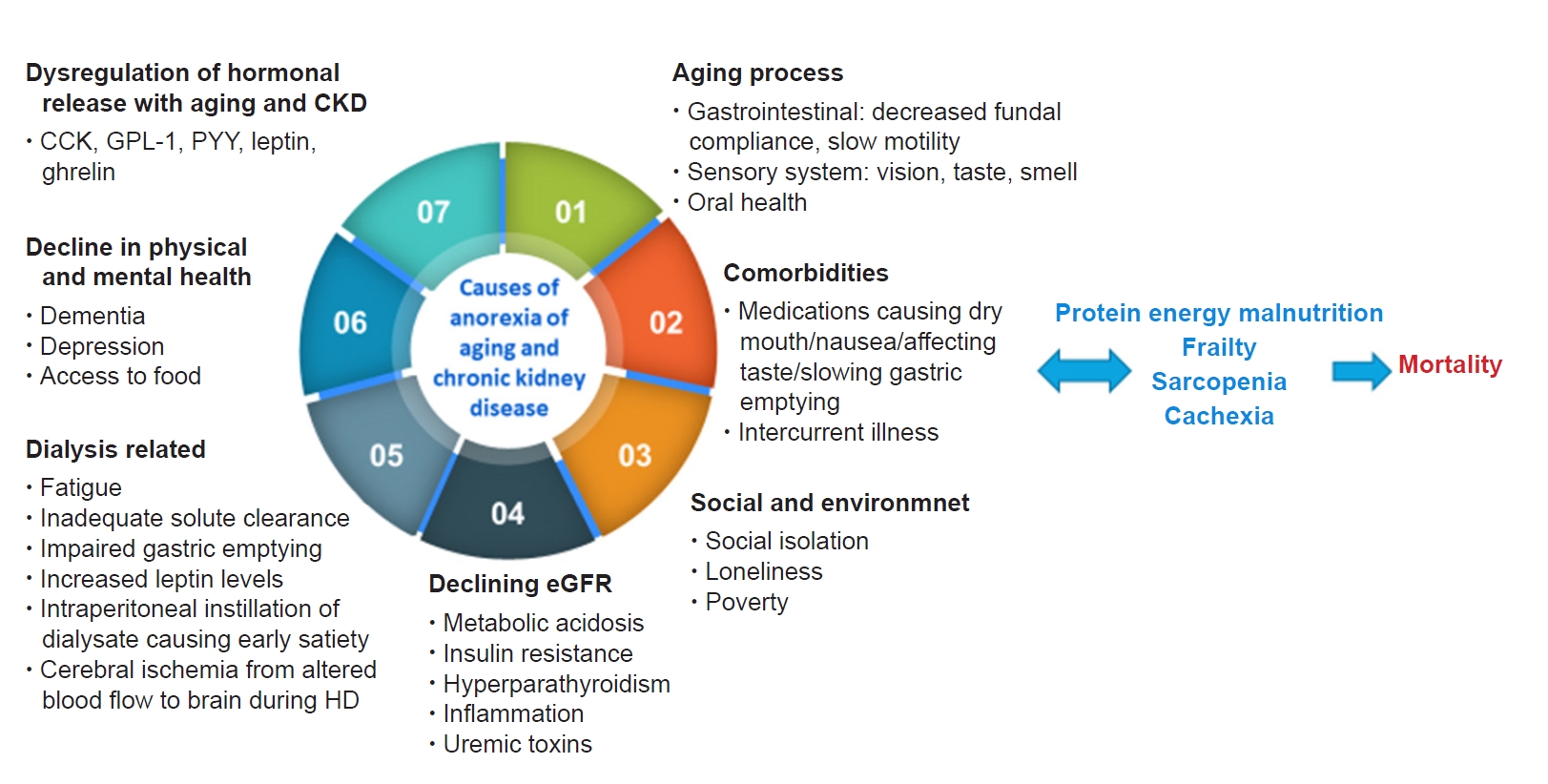
Causes of anorexia associated with aging or due to CKD.
CCK, cholecystokinin; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; GLP-1, glucagon-like peptide-1; HD, hemodialysis; PYY, peptide YY.
The commonly used tools in studies and clinical practice for the evaluation of anorexia or loss of appetite are listed in Table 3 [58–64]. The Simplified Nutrition Assessment Questionnaire (SNAQ) is a simplified version of the Council on Nutrition Appetite Questionnaire that is simple to use, has been validated worldwide and was shown to be associated with depression, number of medications, poor self-rated health, and eating alone [59,60,65]. The prevalence of AA is 12% to 70% depending on the tools used and the participants studied. Among patients with CKD, the prevalence of anorexia is between 35% and 50%, and it is significantly associated with increasing age, poor QoL, and mortality [66,67].
Person-centered care in older patients with chronic kidney disease
With growth of the aging population, the number of older patients with CKD will continue to increase. The aging CKD population with/without dialysis will have an increased prevalence of loss of appetite, sarcopenia, frailty, cognitive impairment, and PEM, which are all associated with poor outcomes including hospitalization, disability, and mortality [11]. Healthcare systems across the world need to have systems in place for the prevention of CKD amongst high-risk older adults (e.g., diabetics), screening for poor prognostic factors amongst patients with CKD (e.g., frailty, poor appetite, cognitive impairment) and provide necessary interventions to reverse underlying factors that may contribute to poor outcomes.
Screening for frailty and cognitive impairment will enable us to manage patients with CKD better with shared decision-making where treatment options such as dialysis may prolong life but cause significant physical and psychological burdens and poor QoL. In other situations, frailty could be reversed or optimized before kidney transplantation, although this may lead to patients not being put on the transplant list. With limited numbers of geriatricians worldwide, close collaboration between geriatricians and nephrologists is required to implement assessment (e.g., comprehensive geriatric assessment) and interventions. The Rapid Geriatric Assessment tool (Fig. 3), which is available in the Epic electronic health records (https://www.epic.com/about) and as an app to screen for frailty (FRAIL), sarcopenia (SARC-F), AA (SNAQ) and cognition (Rapid Cognitive Screen) with an assisted management pathway, may be useful in centers with limited geriatric resources [45,68]. While several intervention strategies have been shown to be useful, two key approaches that warrant mention include nutrition and exercise training.
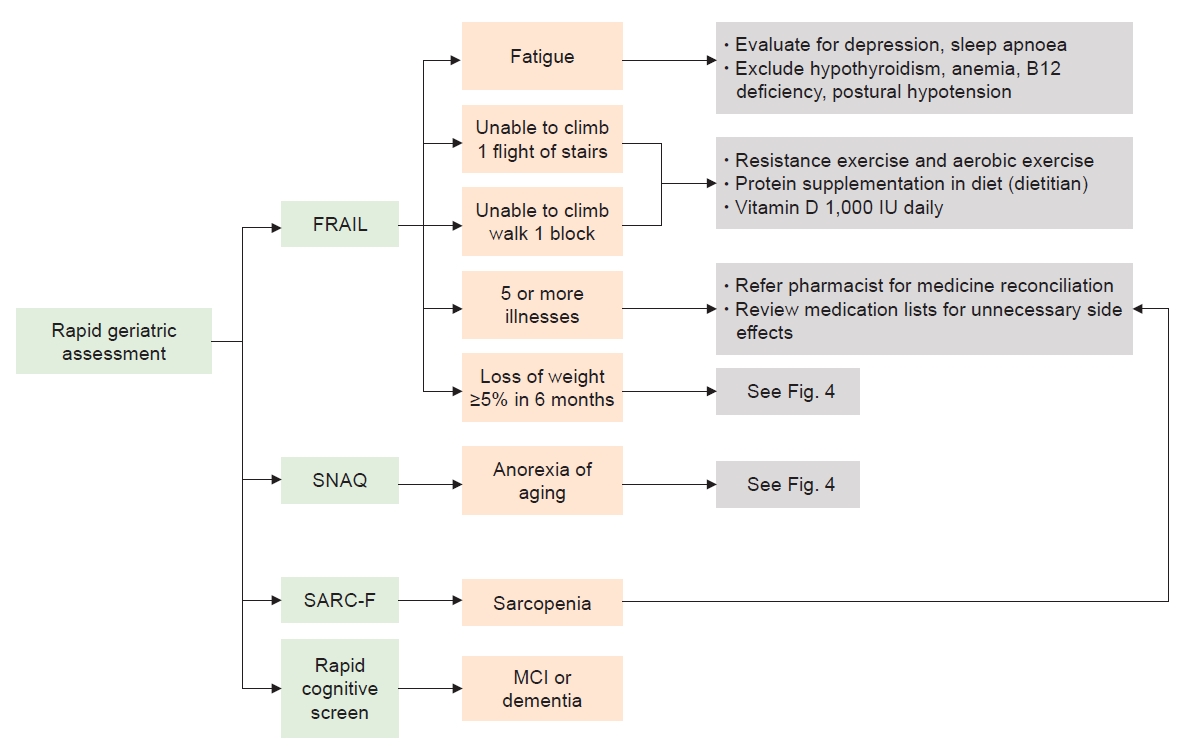
Rapid geriatric assessment with an assisted management pathway.
MCI, mild cognitive impairment; SARC-F, strength, ambulation, rising from a chair, climbing stairs, and falls questionnaire; SNAQ, Simplified Nutrition Assessment Questionnaire.
Nutrition
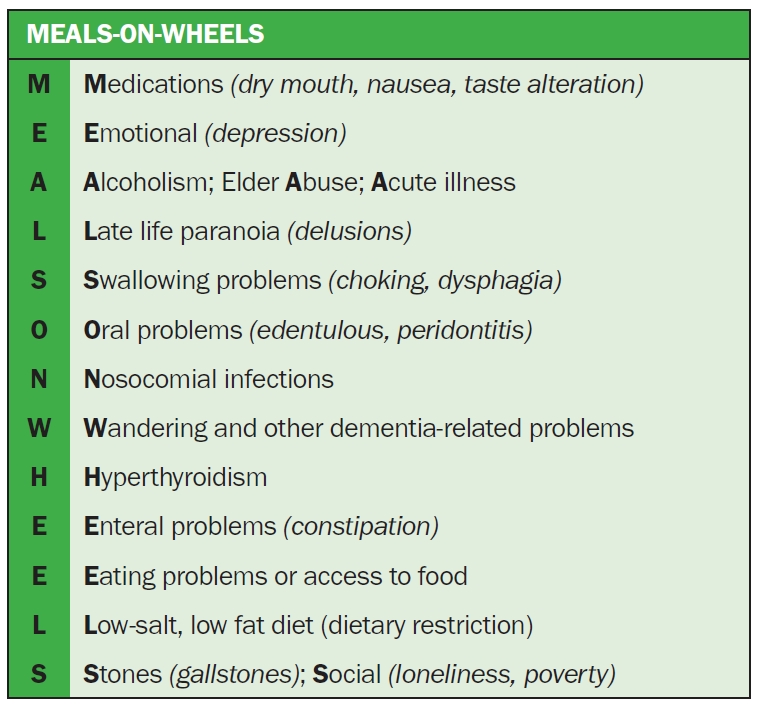
The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (KDOQI) continues to revise clinical practice guidelines on the management of nutrition based on new evidence and to focus on six key areas: nutritional assessment, medical nutrition therapy, protein and energy intake, nutritional supplementation, micronutrients, and electrolytes [69]. A loss of appetite requires evaluation to exclude treatable or reversible factors based on the mnemonic ‘Meal on Wheels,’ such as medications, emotion (e.g., depression) and alcohol/substance or elder abuse, etc., as shown in Fig. 4.
For protein intake in metabolically stable CKD stage 3 to 5 patients, the KDOQI recommends a low-protein (0.55–0.60 g of dietary protein/kg body weight/day) or very-low-protein (0.28–0.43 g of dietary protein/kg body weight/day) diet with additional keto acid/amino acid analogs to meet protein intake needs. A protein intake of 0.6 to 0.8 g/kg body weight per day is recommended for CKD stage 3 to 5 patients with diabetes. For those on dialysis, regardless of diabetes, a dietary protein intake of 1.0 to 1.2 g/kg body weight per day is recommended [69]. A prescription of energy intake of 25 to 35 kcal/kg body weight per day is recommended in CKD stage 1 to 5D to maintain nutritional status [69]. Oral nutritional solutions in non-dialysis and dialysis patients with CKD have been shown to improve nutrition and inflammation [70,71]. The KDOQI recommends a minimum of a 3-month trial of oral nutritional supplements to improve dietary status in those who failed to respond to dietary counseling to meet nutritional requirements.
The role of appetite stimulants like megestrol acetate requires further evaluation in larger randomized control trials [72]. As the aging population and those with CKD are very heterogeneous, personalized assessments, which assess psychosocial aspects, environment, comorbidities with associated polypharmacy, frailty, and dementia, are necessary before providing intervention for loss of appetite, malnutrition, and/or PEM. Poor dietary compliance is often caused by contradictory and complicated dietary regimes without taking personal preference into account [73]. For those in a hypercatabolic state, malnourished and/or frail, there should be some flexibility in dietary recommendations.
Exercise
Exercise is considered a poly-pill for primary prevention and a multitude of medical diagnoses, including frailty and dementia, where no cure is available [20]. Older patients with CKD have multimorbidity’s, poor effort tolerance, fatigue, poor QoL, and poor appetite and are at increased risk of social isolation. While renal rehabilitation includes recommendations on diet and water management, pharmacotherapy, education, and psychological support, exercise is a core tenet of renal rehabilitation [74]. Insufficient physical activity increased with disease progression and increasing age in CKD patients [75]. While 34% of CKD stage 1 to 2 patients were physically active, only 11% of CKD stage 5, 6% of HD patients, and 8% of PD patients were physically active in the same study [75]. Physical inactivity is one of the causes of a high symptom burden and leads to a loss of muscle mass and strength with a consequent functional decline, disability, poor QoL, and mortality [76].
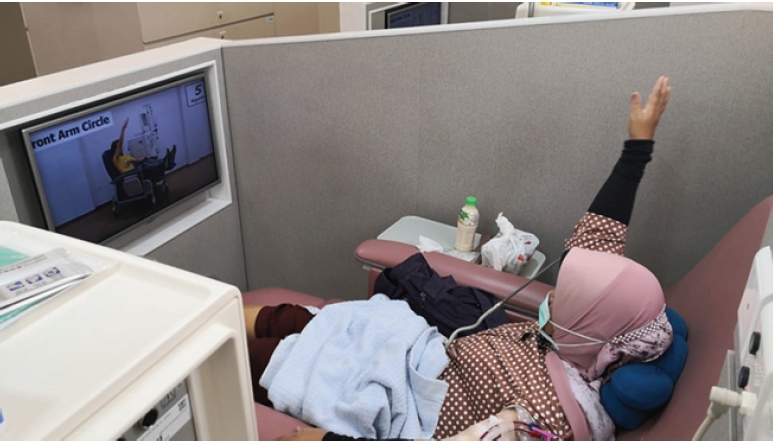
Exercise is beneficial in both CKD non-dialysis and CKD dialysis patients, being partially possibly mediated through a muscle-bone crosstalk mechanism [77]. The DOPPS (Dialysis Outcomes and Practice Pattern Study) showed that CKD patients on maintenance HD who participated in aerobic physical activity had better QoL, survival and fewer depression symptoms [78]. Moraes et al. [79] found that 6 months of resistance exercise increased physical function (as assessed by the 36-Item Short Form Survey), plasma concentrations of appetite hormones (acyl-ghrelin), body composition, and nutritional status in HD patients. Intradialytic exercise (Fig. 5) is increasingly encouraged in dialysis centers worldwide as it is associated with increased dialysis efficacy, better QoL, and improved physical function [80,81]. Specific exercises need to be prescribed and must include aerobic, strength, and flexibility exercises performed 2 to 3 days/wk. Each exercise should have ≤2 sets of 12 to 15 reps, and common modalities include cycle ergometer, weights/resistance bands, and static stretching [82].
Systematic reviews on the benefits of exercise in pre-dialysis patients showed that it improved eGFR, blood pressure, body mass index, inflammatory markers, maximal oxygen uptake peak, and QoL [83–85]. A lower decline in glomerular filtration and improvements in muscle strength were reported in a 12-week randomized study combining resistance exercise with a low-protein diet by Castaneda et al. [86]. While there is limited evidence for dual-task exercise in CKD patients, it has been shown to improve gait speed, cognition, pain, and perceived health in at-risk community-dwelling older adults [87].
Future studies
The literature on frailty in older patients with CKD has increased significantly in the last 5 years, but significant gaps remain, which warrants further research. Despite a large number of frailty screening tools, there is still a lack of optimal screening tools in patients with CKD. Most researchers and dialysis centers worldwide are using physical performance tools, such as the sit-to-stand test or the gait speed test, which allow objective comparisons across different time points. Given the reversibility of frailty and pre-frailty in the general population, it remains unclear whether interventions to alter the trajectory of frailty, such as nutrition and exercise, will help to slow CKD progression. Future studies should focus on the role of various different types of intervention in delaying the onset of frailty in pre-dialysis or dialysis patients. There is very little literature on the impact of frequency or dosage of dialysis and reducing HD complications on frailty and cognitive impairment trajectory. Lastly, we do not know if physical activity and nutrition intervention in the peri-dialysis initiation period will have an overall impact on outcomes like mortality and functional decline after the initiation of dialysis.
Conclusion
CKD is a model for accelerated aging and is significantly associated with frailty, cognitive impairment, and loss of appetite. Early screening with necessary interventions, such as exercise and nutrition interventions, may help to modify the trajectory of CKD and improve physical function, cognition, and perceived health.
Notes
Conflicts of interest
All authors have no conflicts of interest to declare.
Authors’ contributions
Conceptualization: RAM, AV
Writing–original draft: RAM
Writing–review & editing: RAM, AV
All authors read and approved the final manuscript.