Introduction
Venous thrombosis is one of the major life-threatening complications of nephrotic syndrome. The pathophysiology of thrombogenesis is not fully understood yet and the risk of venous thrombotic complications varies according to age, histologic type of renal disease, and level of serum albumin. Deep venous thrombosis, renal vein thrombosis, and pulmonary embolism are common in nephrotic syndrome, whereas portal vein and superior mesenteric vein (SMV) thrombosis in nephrotic syndrome are rare. There were several cases of portal vein thrombosis reported as a complication of nephrotic syndrome treated with anticoagulation therapy alone. Here, we describe a case of portal, splenic, and SMV thrombosis which occurred in a patient with a relapsed steroid dependent minimal change disease (MCD) and who was treated with anticoagulation and thrombolytic therapy.
Case report
A 31-year-old man presented to our hospital because of severe diffuse abdominal pain and vomiting for the past 2 days. He was diagnosed with MCD, which was confirmed by renal biopsy 4 years ago. He had been treated with corticosteroid and cyclosporine 4 years ago and then stopped the corticosteroid treatment when complete remission was achieved. However, relapse of nephrotic syndrome occurred soon, and his medication was changed to tacrolimus and corticosteroid. After achieving complete remission again, he had been maintained with tacrolimus. He had diffuse abdominal pain without diarrhea, melena, or hematochezia. In addition, he had no history of previous abdominal surgery and trauma. He was an ex-smoker (5 pack-year) and he quit smoking 4 years ago. On physical examination, his body temperature was 36.8°C, blood pressure 140/70 mmHg, respiratory rate 26 breaths/min, and heart rate 102 beats/min. He gained weight of about 3 kg in 1 month. There was diffuse and rebound tenderness on the abdomen and facial edema and moderate pitting edema on lower limbs. Initial laboratory data showed a relapsing nephrotic syndrome. Total protein was 4.4 g/dL and serum albumin was 1.6 g/dL. His total cholesterol was 489 mg/dL, and triglyceride was 270 mg/dL. The urinalysis showed an isolated proteinuria and urine protein creatinine ratio of 4,353 mg/g. Other laboratory results were as follows: hemoglobin of 19.0 g/dL, hematocrit of 54.0%, white blood cells of 10,280/mm3 and platelets of 157,000/mm3. The blood urea nitrogen was 19.3 mg/dL and creatinine was 0.96 mg/dL. The serum concentration of C-reactive protein was 1.75 mg/dL.
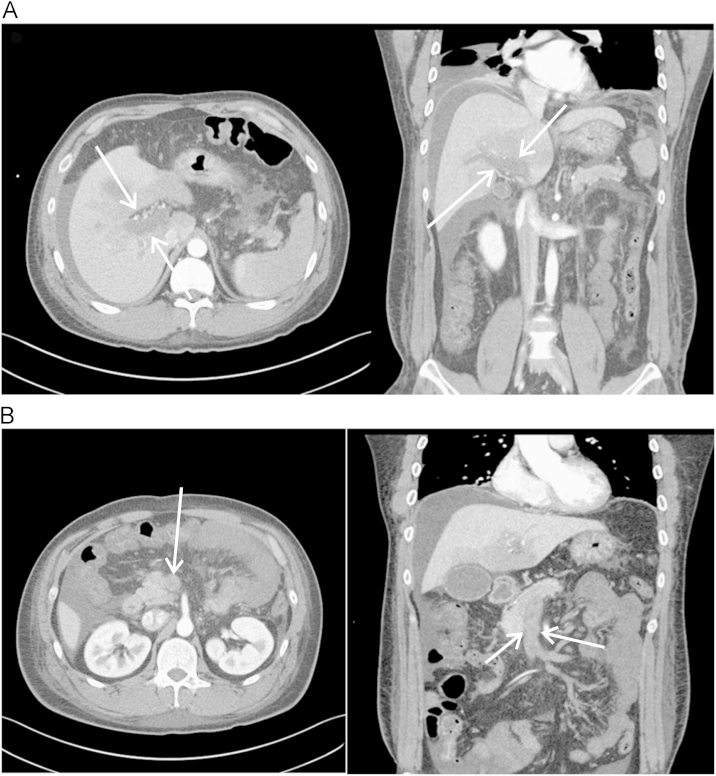
Enhanced abdomen computed tomography (CT) scan was performed and showed diffuse portal, splenic, and SMV thrombosis accompanied with bowel edema probably due to bowel ischemia (Fig. 1). We evaluated the coagulation status with prothrombin time, activated partial thromboplastin time, D-dimer, fibrinogen, protein C, protein S, antithrombin III, antiphospholipid antibody, and the factor V Leiden mutation. His prothrombin time was 11.6 seconds, INR 1.03 (international normalized ratio, normal range: 0.85–1.30), the activated partial thromboplastin time was 36.4 seconds and the antithrombin III was 48.6% (normal range: 80–120%). Protein C showed 163.2% (normal range: 72–150%) and protein S 79.0% (normal range: 72–150%). Antiphospholipid antibody and factor V Leiden mutation were negative.
An intravenous infusion of unfractionated heparin was immediately initiated as an anticoagulation therapy. Urokinase was injected via selective catheterization of the superior mesenteric artery (SMA) as thrombolytic therapy at the same time. An angiography showed no visualization of the portal vein, SMV, and splenic vein. The catheter was placed on the tip in the SMA and a continuous thrombolytic therapy was started with urokinase 50,000 IU/h. During the prolonged infusion of urokinase, the follow-up SMV venography via the infusion catheter was performed every 24 hours for 5 days. There was no visualization of the main portal vein and SMV, but collateral vessel formation was observed at the follow-up SMV venography and symptoms were gradually improved. Thrombolytic therapy was stopped and anticoagulation therapy with heparin was switched to warfarin.
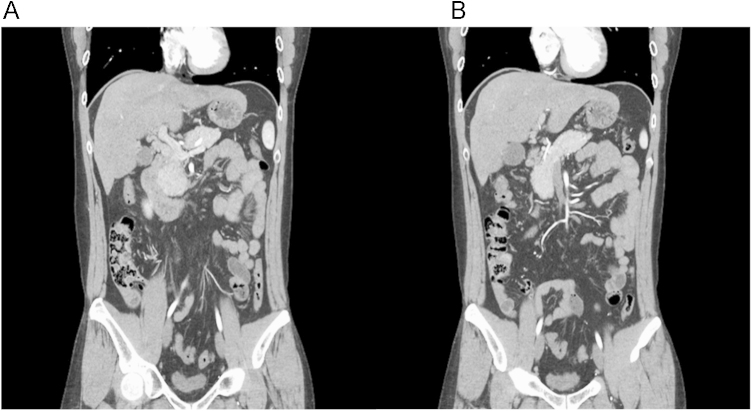
While anticoagulation with thrombolytic therapy was on-going, we did not control the underlying nephrotic syndrome because of infection risk and other complications related to ischemic colitis. We started mycophenolate mofetil (1,000 mg/d) and 1 mg/kg of prednisolone to control nephrotic syndrome when the patient could eat food and was symptom free with regards to his abdomen. Six months later, complete remission (reduction of proteinuria to <300 mg/g) of the nephrotic syndrome was maintained and the follow-up CT scan showed disappearance of all portal vein, SMV, and splenic vein thrombi (Fig. 2).
Discussion
Venous thrombosis is a common and serious complication in nephrotic syndrome. About 25% of nephrotic syndrome patients suffer from systemic thromboembolic complications [1]. It has been known why thromboembolic complications increase in patients with nephrotic syndrome; the alterations in coagulation factors, fibrinolysis, and platelet function lead to a hypercoagulability in nephrotic syndrome. In addition, low levels of plasma albumin, dyslipidemia, and the therapeutic use of diuretics and corticosteroids are thought to contribute to the formation of thrombi [2], [3].
SMV thrombosis is an uncommon and potentially lethal disease because its presenting symptoms overlap with those of many other diseases, leading to significant delays in diagnosis and therapy. Anticoagulation therapy alone could be sufficient in a mild form of portal and SMV thrombosis. The main treatments of portal and SMV thrombosis are anticoagulation and thrombolytic therapy and these should be considered and started as soon as possible after diagnosis. Thrombolytic therapy is a safe and effective method for patients with symptomatic acute portal vein and SMV thrombosis, and urokinase is currently the most established thrombolytic agent for venous thrombosis [4], [5]. Thrombolytic therapy can be administered directly via percutaneous-transhepatic or transjugular-transhepatic routes or indirectly via SMA infusion of thrombolytic agents. Direct thrombolytic therapy is more efficient and decreases the dose of the thrombolytic agent, lowering the risk of related complications. However, an indirect method is technically easy compared to a direct method and there are potential benefits in infusing thrombolytic agents into small mesenteric venous branches [6].
Venous thrombosis is a common complication of nephrotic syndrome. However, the beneficial effect of routine primary prophylactic anticoagulation is uncertain. Advanced age, membranous nephropathy, severe proteinuria, hypovolemia, hypoalbuminemia, and dyslipidemia are all now recognized to be correlated with an increased risk of thromboembolism development. Therefore, primary prophylactic anticoagulation needs to be carefully considered for such high-risk patients and an acute mesenteric venous thrombosis should be suspected when severe abdominal pain, vomiting, and ascites develop in patients with nephrotic syndrome [7]. In this case, although the patient had recurrent episodes of relapse, he was young and had states of normoalbuminemia, euvolemia, and mild proteinuria as an outpatient. Therefore, we did not give prophylactic anticoagulation.
In our case, the patient’s abdominal pain continued and worsened. However, there was no evidence of bowel perforation or necrosis. We therefore immediately started anticoagulation therapy and performed thrombolytic therapy simultaneously. An immediate surgical intervention is needed if the patient is suspected of having intestinal infarction and necrosis [4]. The indirect thrombolytic therapy via SMA was successfully administered and the patient’s symptoms improved, albeit the thrombi remained. In SMA angiography, regression of the thrombi was not observed, however, thrombolytic therapy prevents the progression of a thrombus formation and helps to develop a collateral vessel formation that prevents the progression of bowel ischemia. Six months later, we discontinued the anticoagulation, because a complete remission of the nephrotic syndrome was maintained and the thrombi had disappeared.
In summary, the possibility of an acute portal vein or SMV thrombosis should be considered when severe acute abdominal pain and nausea occur in patients with nephrotic syndrome, especially in high risk patients. Anticoagulant therapy with thrombolytic therapy should be considered and provided when bowel ischemia or infarction is suspected. Interventional treatment, including direct or indirect portal vein and SMV thrombolysis, is a safe and effective strategy for patients with symptomatic acute portal vein and SMV thrombosis prior to the development of bowel infarction.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















