| Kidney Res Clin Pract > Volume 33(4); 2014 > Article |
|
Abstract
Background
Recent evidence demonstrates that high doses of epoetin-alpha (EPO-╬▒) can be administrated at extended intervals, despite its relatively short serum half-life. However, no prospective randomized trials on the effects of extended dosing intervals of EPO-╬▒ compared with darbepoetin-alpha (DA-╬▒) have been performed. This study was designed to investigate whether a single biweekly (Q2W) administration of a high dose of EPO-╬▒ is as effective as DA-╬▒ for anemia in chronic kidney disease (CKD) patients not receiving dialysis.
Methods
Sixty non-dialysis CKD patients were equally randomized to either Q2W subcutaneous EPO-╬▒ (10,000 unit) or DA-╬▒ (50┬Ā╬╝g) therapy groups for the first 6 weeks. After a 6-week washout period, the participants of the EPO-╬▒ and DA-╬▒ treatment groups switched to the alternate regimen for 6 weeks. The mean hemoglobin (Hb) levels after erythropoiesis stimulating agent (ESA) therapy and percentage change in Hb levels from baseline to the end of the study were analyzed.
Results
The mean Hb levels of postESA therapy increased significantly compared with those of preESA therapy in both ESA regimens. The percentage increase in Hb levels and erythropoietin resistance index did not show a significant difference between the different ESA regimens. No difference was observed between the regimens regarding mean Hb levels after ESA therapy. Additionally, there were no serious adverse effects leading to withdrawal from treatment.
Conclusion
Biweekly high doses of EPO-╬▒ therapy may be equally as effective as Q2W DA-╬▒ therapy in maintaining target Hb levels in non-dialysis CKD patients.
Keywords
Anemia, Chronic kidney disease, Darbepoetin-alpha, Epoetin-alphaAnemia is a well-known, independent risk factor for cardiovascular disease in patients with chronic kidney disease (CKD) [1], [2], [3]. If left untreated, the anemia of CKD can result in the deterioration of cardiac function, decreased cognition, mental acuity, fatigue, and other signs and symptoms. Furthermore, anemia of CKD is associated with an increased risk of morbidity and mortality, principally due to cardiac disease and stroke [4], [5]. Consequently, anemia remains a significant problem in patients with CKD who do not yet require dialysis.
Correction of renal anemia with erythropoiesis-stimulating agents (ESAs) in non-dialysis CKD patients may provide potential benefits including the improvement of patient outcomes. [4], [5], [6], [7], [8]. Thus, it is important to manage renal anemia using ESAs, including short-acting ESAs, darbepoetin-alpha (DA-╬▒), and methoxy polyethylene glycol-epoetin-beta (continuous erythropoiesis receptor activator, CERA), in non-dialysis CKD patients. However, there is no evidence that any one ESA is superior to another in terms of patient outcomes. Therefore, the 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guideline for Anemia in CKD recommended choosing an ESA based on a number of different aspects such as the balance of pharmacodynamics, safety information, clinical outcome data, cost, and availability [5].
The frequency of ESA administration should be determined based on CKD stage, treatment setting, efficacy considerations, patient tolerance and preference, and type of ESA [5]. The need for frequent dosing of short-acting ESAs can be a considerable burden on both patients and health care staff, and it may contribute to the undertreatment of anemia in nondialysis CKD patients. Because undertreatment of anemia may contribute to poor outcomes, patientsŌĆÖ compliance is not a minor problem in managing anemia in nondialysis CKD patients. In terms of patientsŌĆÖ compliance, long-acting ESAs such as DA-╬▒ and CERA have some advantages over short-acting epoetin-alpha (EPO-╬▒) [9], [10].
The longer half-life enables DA-╬▒ to effectively maintain target hemoglobin levels with less frequent dosing. The extended dosing interval of once weekly or once every 2 weeks offers many potential benefits to both patients and health care staff [11], [12]. In comparison, the efficacy of EPO-╬▒ decreases when the dosing intervals are extended to a once-weekly or biweekly administration. However, there is increasing evidence that a higher dose of EPO-╬▒ can be administrated at extended dosing intervals while maintaining its efficacy, despite its relatively short serum half-life [13], [14], [15], [16]. Our previous open-label, single-arm study also showed the beneficial effect of high dose EPO-╬▒ at extended intervals in nondialysis CKD patients [17]. However, to date, there have been no randomized, crossover trials comparing a single biweekly administration of high EPO-╬▒ with DA-╬▒ in nondialysis CKD patients.
Therefore, we designed a randomized study to investigate whether a single biweekly high dose of EPO-╬▒ is as effective as a single biweekly equivalent dose of DA-╬▒ in correcting anemia in nondialysis CKD patients.
This study was conducted as an open-label, prospective, randomized, crossover trial. Adult nondialysis CKD patients were recruited from three hospitals between May 2009 and December 2009. The inclusion criteria were: (1) adult CKD patients (Ōēź18 years) who did not require dialysis; (2) glomerular filtration rate (GFR) Ōēż60┬ĀmL/min/1.73┬Ām2, calculated by using the abbreviated Modification of Diet in Renal Disease (MDRD) Study equation; (3) hemoglobin (Hb) Ōēż11┬Āg/dL; (4) adequate iron status: serum ferritin Ōēź100┬Āng/mL or transferrin saturation (TSAT) Ōēź20%; and (5) patients not receiving ESAs for at least 6 weeks prior to randomization. Patients who had any of the following criteria were excluded: (1) nonrenal cause of anemia; (2) pure red cell aplasia; (3) RBC transfusions within the 8 weeks prior to the study; (4) current active malignancy; (5) poorly controlled diabetes (HbA1C >10.0%); (6) poorly controlled hypertension (systolic blood pressure, SBP >170┬ĀmmHg); (7) intact parathyoid hormone (i-PTH) >500┬Āpg/mL; (8) congestive heart failure (NYHA class IV); or (9) pregnancy. Patients were withdrawn from the study if there were any serious adverse effects during the study period; if the patient requested withdrawal; if the ESA dose was missed>25% of a scheduled dose; or if the Hb increased>12.0┬Āg/dL.
The study was approved by the Institutional Review Board of the Konkuk University Medical Center (IRB approval number: KUH1010126). After informed consent had been obtained, eligible patients were equally randomized to receive either a single biweekly (Q2W) subcutaneous DA-╬▒ (50┬Ā╬╝g) therapy (Group 1) or Q2W subcutaneous EPO-╬▒ (10,000 unit) therapy (Group 2) for the first 6 weeks (ESA period-1). After a 6-week washout period, patients switched to the alternate ESA regimen for an additional consecutive 6 weeks (ESA period-2). Laboratory and patient data were collected monthly during the study period. Final data collections were performed at 20 weeks (Fig. 1).
ESAs used in this study were Espogen (10,000 unit, prefilled syringe; LG Life Sciences Inc., Seoul, Korea) and Aranesp (50┬Ā╬╝g, prefilled syringe; Amgen Inc., Thousand Oaks, CA, USA). The dose of EPO-╬▒ was determined based on the standard conversion rate, 200┬ĀIU of EPO-╬▒ to 1┬Ā╬╝g of DA-╬▒ [18], [19].
The primary efficacy endpoint was the mean Hb level after ESA therapy. In addition, the efficacy analysis also compared the percent change of Hb concentration from the baseline and the end of evaluation periods and the erythropoietin resistance index (ERI), calculated as the weekly weight-adjusted dose of erythropoietin (U/kg/week) divided by Hb concentration (g/dL). [20], [21].
The sample size was calculated to compare the erythropoietic effects of a single biweekly dose of EPO-╬▒ with those of DA-╬▒. It was estimated that a sample size of ~65 participants would provide 80% power to demonstrate that a single biweekly EPO-╬▒ dose is similarly effective to DA-╬▒ at an overall 2-sided significance level of 0.05 (95.0% CI).
Statistical analysis was performed using SPSS for Window version 18.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean┬▒standard deviation. The differences between groups were examined using the Student t test for nonpaired samples. Changes from baseline to the last follow-up were compared using the paired t test. Values of P<0.05 were considered to indicate statistical significance.
A total of 74 individuals were assessed for eligibility and 14 of these failed to meet the eligibility criteria. A total of 60 participants were randomized to either a single biweekly dose of DA-╬▒ (Group 1, n=31) or a single biweekly dose of EPO-╬▒ therapy (Group 2, n=29). A total of 54 (90.0%) individuals completed the study and six (10.0%) withdrew (Fig. 2). No statistically significant differences were observed in baseline demographics, clinical characteristics, and laboratory values, including Hb levels, serum ferritin, transferrin saturation, high sensitivity C-reactive protein (hs-CRP), and i-PTH, between the groups (Table 1).
Fig. 3 shows the changes of Hb concentration from baseline to the end of ESA therapy during the study period. In the ESA period-1, Hb levels of postESA therapy increased significantly compared with those of preESA therapy in both groups (Group 1: preDA-╬▒ vs. postDA-╬▒, 9.5┬▒0.9┬Āg/dL vs. 10.8┬▒1.2┬Āg/dL, P<0.001; Group 2: preEPO-╬▒ vs. postEPO-╬▒, 9.6┬▒0.9┬Āg/dL vs. 10.3┬▒1.3┬Āg/dL, P=0.002). There was no statistically significant difference in the mean Hb levels of postESA therapy between the two groups in the ESA period-1 (postDA-╬▒ vs. postEPO-╬▒, 10.8┬▒1.2┬Āg/dL vs. 10.3┬▒1.3 g/dL, P=0.052). The percentage increase in Hb levels after ESA therapy also did not show a significant difference between the ESA regimens (DA-╬▒ vs. EPO-╬▒, 14.0 ┬▒ 15.6% vs. 8.4 ┬▒ 9.9%, P=0.072) (Fig. 4). The erythropoietin resistance index also did not show a significant difference between the two groups during the ESA period-1 (DA-╬▒ vs. EPO-╬▒, 8.0┬▒1.7┬ĀIU/kg weight/g Hb vs. 7.9┬▒1.8┬ĀIU/kg weight/g Hb, P=0.744).
However, during the washout period, Hb levels decreased significantly in both groups, but no significant difference was observed between the groups for the mean Hb levels at the end of the washout period (Group 1 vs. Group 2, 9.8┬▒0.9┬Āmg/dL vs. 9.6┬▒0.9 mg/dL, P=0.291). There was also no difference in the percentage decrease in Hb levels during the washout period (Group 1 vs. Group 2, ŌłÆ7.0┬▒11.0% vs. ŌłÆ7.7┬▒9.0%, P=0.808; Fig. 4).
After the washout period, the mean Hb levels of postESA therapy increased significantly compared with those of preESA therapy in both groups during the ESA period-2 (Group 1: preEPO-╬▒ vs. postEPO-╬▒, 9.8┬▒0.9┬Āg/dL vs. 10.3┬▒1.2┬Āg/dL, P<0.001; Group 2: preDA-╬▒ vs. postDA-╬▒, 9.6┬▒0.9┬Āg/dL vs. 10.7┬▒1.2┬Āg/dL, P<0.001). There was no statistically significant difference in Hb levels postESA therapy between the two groups in the ESA period-2 (postDA-╬▒ vs. postEPO-╬▒, 10.5┬▒0.9 g/dL vs. 10.4┬▒1.2┬Āg/dL, P=0.739; Fig. 4). The percentage increase in Hb levels after ESA therapy also did not show a statistically significant differences between the groups (DA-╬▒ vs. EPO-╬▒, 11.4 ┬▒ 10.4% vs. 6.3 ┬▒ 9.1%, P=0.067). In addition, the two groups had similar values of the erythropoietin resistance index during the ESA period-2 (DA-╬▒ vs. EPO-╬▒, 7.6┬▒1.9┬ĀIU/kg weight/g Hb vs. 8.3┬▒2.0┬ĀIU/kg weight/g Hb, P=0.181).
Overall, the mean Hb levels of postESA therapy increased significantly compared with those of preESA therapy in both ESA regimens (DA-╬▒, 9.5┬▒0.9┬Āg/dL vs. 10.7┬▒1.2┬Āg/dL, P<0.001; EPO-╬▒, 9.6┬▒0.9┬Āg/dL vs. 10.3┬▒1.2┬Āg/dL, P=0.002). At 20 weeks, the mean end of study Hb levels were similar in both ESA regimens (postDA-╬▒ vs. postEPO-╬▒, 10.7┬▒1.2┬Āg/dL vs. 10.3┬▒1.2┬Āg/dL, P=0.104). The percentage increase in Hb levels also did not show a significant difference between the two ESA regimens (postDA-╬▒ vs. postEPO-╬▒, 12.7 ┬▒ 13.5% vs. 7.3 ┬▒ 9.8%, P=0.489). Both ESA regimens also had similar values of the erythropoietin resistance index (DA-╬▒ vs. EPO-╬▒, 7.9┬▒1.8┬ĀIU/kg weight/g Hb vs. 8.0┬▒1.9┬ĀIU/kg weight/g Hb, P=0.683).
Of the 60 participants, 32 (53.3%) received oral iron during the study. Most participants were able to maintain adequate iron stores. Mean baseline and end of study ferritin and TSAT were similar in both groups (Table 2).
The adverse event profile was similar in both ESA regimens. A total of 10 (16.6%) participants who received DA-╬▒ and 12 (20.0%) participants who received EPO-╬▒ experienced at least one adverse event during the study period. There were no adverse events serious enough to cause withdrawal of treatment during the study period (Table 3).
The current study showed that an extended dosing schedule consisting of a single biweekly high dose of EPO-╬▒ was well-tolerated and was as effective as a single biweekly dose of DA-╬▒ in maintaining Hb levels in CKD patients who do not require dialysis.
Despite a short serum half-life, which is ~30 hours when administrated subcutaneously, a single biweekly administration of EPO-α can effectively increase Hb in nondialysis CKD patients [13], [22], [23]. A number of prospective studies have demonstrated the effectiveness and safety of high dose short-acting ESA with extended dosing intervals. In the Clinical Evaluation of PROCRIT® for Maintenance Phase Treatment of Patients With Anemia Due to Chronic Kidney Disease (PROMPT) study, 519 CKD patients with renal anemia (baseline Hb >11 g/dL) were randomly assigned to receive 10,000 units, 20,000 units, 30,000 units, or 40,000 units of subcutaneous EPO-α every week, 2 weeks, 3 weeks, and 4 weeks, respectively. At the end of the 16-week treatment period, the mean Hb levels were not significantly statistically different and remained above 11 g/dL in all four groups [24]. Another study reported that an EPO-α dose of 40,000 units every 4 weeks was not inferior to EPO-α dose of 20,000 units every 2 weeks as initial therapy in CKD patients with anemia [25].
In terms of patient outcomes, the crux of the matter is that the extended dosing intervals usually require a higher dose of EPO-╬▒. Recent studies suggest that higher doses of ESAs, in themselves, may be at least partly responsible for an increased mortality risk in nondialysis CKD patients [26], [27]. Increasing evidence from multiple well-designed prospective randomized trials, the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR), Cardiovascular risk Reduction by Early Anaemia Treatment with Epoetin ╬▓ (CREATE), and Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) trials in predialysis CKD patients suggest that Hb levels approaching the normal range, compared with levels of moderate anemia, are associated with increased risk of adverse outcomes [28], [29], [30]. In the CHOIR trial, an unadjusted analysis showed an increased risk of the primary composite endpoint (death, myocardial infarction, heart failure, or stroke) at 4 months was associated with both an inability to achieve target levels and higher doses of EPO-╬▒ (>20,000 units per week). However, in an adjusted analysis, only high-dose EPO-╬▒ therapy resulted in an independent increased risk of the primary composite endpoint (HR 1.57, 95% CI 1.04ŌĆō2.36) [28]. These results suggest that an increased mortality may be due to higher ESA doses rather than the higher target Hb level. Therefore, based upon currently available evidence, it is usually recommended that the dose of EPO-╬▒ does not exceed 20,000 units per week in nondialysis CKD patients.
The KDIGO guidelines suggested that ESAs not be started among adult nondialysis CKD patients with Hb concentrations Ōēź10┬Āg/dL and ESAs should generally not be used to maintain Hb concentrations >11.5 g/dL. In addition, the KDIGO guidelines also suggested that ESA therapy should be individualized as some patients may have improvements in quality of life at Hb Ōēź11.5 g/dL. The suggestion to maintain Hb levels in the 10.0ŌĆō11.5 g/dL range in nondialysis CKD patients is based on the interpretation of the results of the recent prospective randomized trials, which found that high Hb targets are associated with adverse outcomes. In the TREAT trial, there was an increased risk of fatal or nonfatal stroke with DA-╬▒ [30]. In the CHOIR trial, a higher Hb target was associated with a greater risk of progression of CKD. In addition, there were a significantly higher number of adverse events in the high Hb group in the CHOIR trial. This difference was primarily due to insignificant trends in the high Hb group, including an increased risk of death and hospitalization for heart failure. In a secondary analysis of the CHOIR trial, the increased risk with higher-dose EPO-╬▒ was observed in both the high- and low-target Hb groups [26]. These results suggest that any adverse effects including an increased mortality may be due to higher ESA doses rather than a higher Hb level.
With respect to the toxic effects of high-dose ESA therapy, the results of our prospective randomized study provided important information: only 5,000 units of EPO-╬▒ per week with a single biweekly subcutaneous administration was as effective as DA-╬▒ in maintaining target Hb levels in nondialysis CKD patients. In the present study, no differences were observed between the groups for the primary efficacy endpoint (mean Hb levels after ESA therapy). Both groups had similar changes in other efficacy endpoints such as the percentage change of Hb concentration between the baseline and evaluation periods and the erythropoietin resistance index.
Our study had several potential limitations. The main limitation is the relatively short period of evaluation. In the present study, the follow-up period to evaluate the efficacy of each ESA regimen was only 8 weeks in the per-protocol population. The period is too short to reveal a sufficient erythropoietic effect of ESA and to demonstrate Hb stability. It has been suggested that at least 16 weeks may be needed to achieve stability of the DA-╬▒ dose and Hb level. Due to the half-life of circulating red blood cells, which is ~60 days in dialysis patients, it is anticipated that the equilibrium of Hb concentrations after switching from EPO-╬▒ to DA-╬▒ would occur within 20ŌĆō24 weeks. However, a recent study demonstrated that the median time to a Hb increase of>1 g/dL from baseline ranged from 3 weeks to 5 weeks after extended dosing with EPO-╬▒ [13]. Thus, we expected that the impact of the extended dosing of EPO-╬▒ and DA-╬▒ would manifest within 8 weeks. The other limitations in our study are the relatively small number of participants and the nonblinded design. However, this study is a randomized, controlled crossover trial, which has some advantages over a parallel and a noncrossover longitudinal study. One of its advantages is that crossover designs are statistically efficient and so require fewer participants than do noncrossover designs. The limitation of the relatively small number of participants is partially overcome by the randomized, controlled crossover design of the present study.
In conclusion, our study demonstrated that a single biweekly high dose of EPO-╬▒ therapy may be as effective as a single biweekly dose of DA-╬▒ therapy in maintaining target Hb levels in nondialysis CKD patients with renal anemia. Further randomized controlled trials are necessary to clarify the long-term clinical benefits and adverse effects of extended dosing of EPO-╬▒ treatment in CKD patients who are not on dialysis.
References
1. Petretta M, Scopacasa F, Fontanella L, Carlomagno A, Baldissara M, de Simone A, Petretta MP, Bonaduce D. Prognostic value of reduced kidney function and anemia in patients with chronic heart failure. J Cardiovasc Med (Hagerstown) 8:2007;909ŌĆō916.


2. Al-Ahmad A, Rand WM, Manjunath G, Konstam MA, Salem DN, Levey AS, Sarnak MJ. Reduced kidney function and anemia as risk factors for mortality in patients with left ventricular dysfunction. J Am Coll Cardiol 38:2001;955ŌĆō962.


3. Goicoechea M, de Vinuesa SG, Gomez-Campdera F, Luno J. Predictive cardiovascular risk factors in patients with chronic kidney disease (CKD). Kidney Int Suppl 93:2005;S35ŌĆōS38.

4. KDOQI ; National Kidney Foundation. KDOQI Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47:2006;S11ŌĆōS145.

5. KDIGO Clinical practice guidelines for anemia in chronic kidney disease. Kidney Int Suppl 2:2012;282ŌĆō335.
6. Revicki DA, Brown RE, Feeny DH, Henry D, Teehan BP, Rudnick MR, Benz RL. Health-related quality of life associated with recombinant human erythropoietin therapy for predialysis chronic renal disease patients. Am J Kidney Dis 25:1995;548ŌĆō554.


7. Jungers P, Choukroun G, Oualim Z, Robino C, Nguyen AT, Man NK. Beneficial influence of recombinant human erythropoietin therapy on the rate of progression of chronic renal failure in predialysis patients. Nephrol Dial Transplant 16:2001;307ŌĆō312.


8. Mohanram A, Zhang Z, Shahinfar S, Keane WF, Brenner BM, Toto RD. Anemia and end-stage renal disease in patients with type 2 diabetes and nephropathy. Kidney Int 66:2004;1131ŌĆō1138.


9. Di Lullo L, Floccari F, Granata A, Malaguti M. Low-dose treatment with erythropoiesis-stimulating agents and cardiovascular geometry in chronic kidney disease: is darbepoetin-╬▒ more effective than expected? Cardiorenal Med 2:2012;18ŌĆō25.


10. Bock HA, Hirt-Minkowski P, Brunisholz M, Keusch G, Rey S, von Albertini B. Darbepoetin alpha in lower-than-equimolar doses maintains hemoglobin levels in stable hemodialysis patients converting from epoetin alpha/beta. Nephrol Dial Transplant 23:2008;301ŌĆō308.


11. Locatelli F, Olivares J, Walker R, Wilkie M, Jenkins B, Dewey C, Gray SJ; European/Australian NESP 980202 Study Group. Novel erythropoiesis stimulating protein for treatment of anemia in chronic renal insufficiency. Kidney Int 60:2001;741ŌĆō747.


12. Carrera F, Burnier M. Use of darbepoetin alfa in the treatment of anaemia of chronic kidney disease: clinical and pharmacoeconomic considerations. NDT Plus 2:2009;i9ŌĆōi17.



13. Benz R, Schmidt R, Kelly K, Wolfson M. Epoetin alfa once every 2 weeks is effective for initiation of treatment of anemia of chronic kidney disease. Clin J Am Soc Nephrol 2:2007;215ŌĆō221.


14. Pergola PE, Gartenberg G, Fu M, Sun S, Wolfson M, Bowers P. A randomized controlled study comparing once-weekly to every-2-week and every-4-week dosing of epoetin alfa in CKD patients with anemia. Clin J Am Soc Nephrol 5:2010;598ŌĆō606.



15. Pergola PE, Gartenberg G, Fu M, Wolfson M, Rao S, Bowers P. A randomized controlled study of weekly and biweekly dosing of epoetin alfa in CKD patients with anemia. Clin J Am Soc Nephrol 4:2009;1731ŌĆō1740.



16. Carrera F, Disney A, Molina M. Extended dosing intervals with erythropoiesis-stimulating agents in chronic kidney disease: a review of clinical data. Nephrol Dial Transplant 22:2007;iv19ŌĆōiv30.


17. Kim SY, Choi HJ, Choi HJ, Lee CE, Yun SU, Park JH, Lee JH, Song JO, Jo YI.. Comparison of once-biweekly administration of epoetin-╬▒ with darbepoetin-╬▒ in chronic kidney disease patients not receiving dialysis. Korean J Nephrol 29:2010;562ŌĆō569.
18. Hirai T, Sugiya N, Nakashima A, Takasugi N, Yorioka N. Switching from epoetin alpha to darbepoetin alpha in Japanese hemodialysis patients: dose conversion ratio. Nephron Clin Pract 111:2009;c81ŌĆōc86.


19. Horowitz J, Agarwal A, Huang F, Gitlin M, Gandra SR, Cangialose CB. Empirical methods to calculate an erythropoiesis-stimulating agent dose conversion ratio in nondialyzed patients with chronic kidney disease. J Manag Care Pharm 15:2009;741ŌĆō750.


20. Regidor DL, Kopple JD, Kovesdy CP, Kilpatrick RD, McAllister CJ, Aronovitz J, Greenland S, Kalantar-Zadeh K. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol 17:2006;1181ŌĆō1191.


21. Perez-Garcia R, Rodriguez Benitez P, Jofre R, Lopez-Gomez JM, Villaverde MT, Blanco A, Blanco S, Sanchez M. Resistance index to epoetin alpha and to darbepoetin-alpha in chronic hemodialysis patients: a cohort study. Nefrologia 27:2007;340ŌĆō349.

22. McGowan T, Vaccaro NM, Beaver JS, Massarella J, Wolfson M. Pharmacokinetic and pharmacodynamic profiles of extended dosing of epoetin alfa in anemic patients who have chronic kidney disease and are not on dialysis. Clin J Am Soc Nephrol 3:2008;1006ŌĆō1014.



23. Piccoli A, Malagoli A, Komninos G, Pastori G. Subcutaneous epoetin-alpha every 1, 2, and 3 weeks in renal anemia. J Nephrol 15:2002;565ŌĆō574.

24. Provenzano R, Bhaduri S, Singh AK; PROMPT Study Group. Extended epoetin alfa dosing as maintenance treatment for the anemia of chronic kidney disease: the PROMPT study. Clin Nephrol 64:2005;113ŌĆō123.


25. Spinowitz B1, Germain M, Benz R, Wolfson M, McGowan T, Tang KL, Kamin M; Epoetin Alfa Extended Dosing Study Group. A randomized study of extended dosing regimens for initiation of epoetin alfa treatment for anemia of chronic kidney disease. Clin J Am Soc Nephrol 3:2008;1015ŌĆō1021.



26. Szczech LA, Barnhart HX, Inrig JK, Reddan DN, Sapp S, Califf RM, Patel UD, Singh AK. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int 74:2008;791ŌĆō798.



27. Keithi-Reddy SR, Addabbo F, Patel TV, Mittal BV, Goligorsky MS, Singh AK. Association of anemia and erythropoiesis stimulating agents with inflammatory biomarkers in chronic kidney disease. Kidney Int 74:2008;782ŌĆō790.



28. Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan D; CHOIR Investigators. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355:2006;2085ŌĆō2098.


29. Dr├╝eke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag A; CREATE Investigators. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355:2006;2071ŌĆō2084.


30. Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto R; TREAT Investigators. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361:2009;2019ŌĆō2032.


Figure┬Ā1
Study design. The eligible patients were equally randomized to receive either a single biweekly subcutaneous darbepoetin-╬▒ (50┬Ā╬╝g) therapy (Group 1) or a single biweekly subcutaneous epoetin-╬▒ (10,000 unit) therapy (Group 2) for the first 6 weeks (ESA period-1). After a 6-week washout period, patients switched to the alternate ESA regimen for the next 6 weeks (ESA period-2). Laboratory and patient data were collected monthly during the study period. DA-╬▒, darbepoetin-╬▒; EPO-╬▒, epoetin-╬▒; ESAs, erythropoiesis-stimulating agents.

Figure┬Ā2
Flow chart of extended dosing schedule of a single biweekly high dose (10,000 units) of epoetin-╬▒ and equal dose (50┬Ā╬╝g) of darbepoetin-╬▒ in nondialysis chronic kidney disease patients. The diagram shows a prospective crossover trial with randomized allocation.

Figure┬Ā3
Change in hemoglobin (Hb) concentration from baseline to the end of ESA therapy during study period. Hb levels of postESA therapy increased significantly compared with those of preESA therapy in both groups. There was no statistically significant difference in the mean Hb levels of postESA therapy between the groups. DA-╬▒, darbepoetin-╬▒; EPO-╬▒, epoetin-╬▒; ESA, erythropoiesis-stimulating agent.
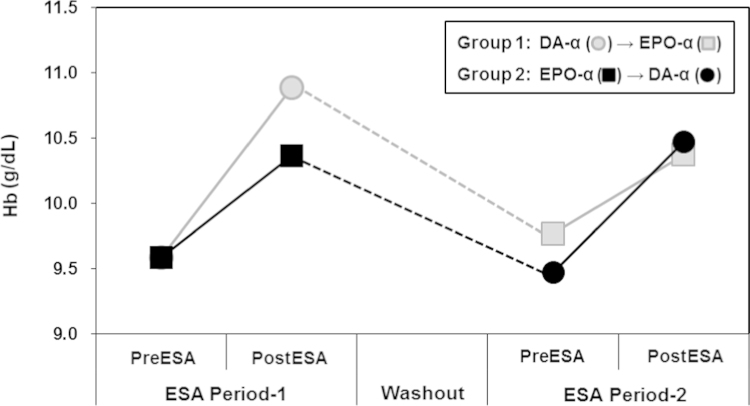
Figure┬Ā4
Percentagechanges in hemoglobin (Hb) levels during study period. The percentage increase in Hb levels after erythropoiesis-stimulating agent (ESA) therapy did not show a significant difference between ESA regimens in ESA period-1 and -2. In addition, there was no difference in the percentage decrease in Hb levels during the washout period.

Table┬Ā1
Demographics, clinical characteristics, and baseline laboratory parameters of the participants
BP, blood pressure; Cr, creatinine; DA-╬▒, darbepoetin-╬▒; eGFR, estimated glomerular filtration rate; e-GFR-MDRD, estimated glomerular filtration rate using Modification of Diet (MDRD) in Renal Disease formula; EPO-╬▒, epoetin-╬▒; hs-CRP, high-sensitivity C-reactive protein; i-PTH, intact parathyroid hormone; SD, standard deviation.
Table┬Ā2
Mean ferritin and TSAT at baseline and end of study
Table┬Ā3
Adverse events during erythropoiesis-stimulating agent therapy in intent-to-treat population



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















