| Kidney Res Clin Pract > Volume 34(3); 2015 > Article |
|
Abstract
A 61-year-old woman was admitted to hospital because of generalized edema and proteinuria. Her renal function deteriorated rapidly. Serum immunoglobulin and complement levels were within normal ranges. An autoantibody examination showed negative for antinuclear antibody and antineutrophil cytoplasmic antibody. Histologic examination of a renal biopsy specimen revealed that all of the glomeruli had severe crescent formations with no immune deposits. The patient was treated with steroid pulse therapy with cyclophosphamide followed by oral prednisolone. Fifteen days later, she experienced massive recurrent hematochezia. Angiography revealed an active contrast extravasation in a branch of the distal ileal artery. We selectively embolized with a permanent embolic agent. On the 45th hospital day, the patient suddenly lost consciousness. Brain computed tomography showed intracerebral hemorrhage. We report a case of antineutrophil cytoplasmic antibody–negative pauci-immune glomerulonephritis with massive intestinal bleeding and cerebral hemorrhage.
Keywords
Gastrointestinal hemorrhage, Glomerulonephritis, VasculitisPauci-immune crescentic glomerulonephritis (GN) is one of the most common causes of rapidly progressive GN (RPGN). Most patients with pauci-immune crescentic GN have glomerular disease as part of an antineutrophil cytoplasmic antibody (ANCA)–associated systemic vasculitis, such as granulomatosis with polyangiitis, microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis, or as a part of renal-limited vasculitis [1]. Because ~75–90% of patients with pauci-immune crescentic GN have circulating ANCA, ANCA is known as a major serologic marker to distinguish pauci-immune crescentic GN from other types of RPGN [2]. Although there is little information about ANCA-negative pauci-immune crescentic GN owing to a relatively small number of patients, several studies have reported fewer systemic vasculitis manifestations in ANCA-negative crescentic GN than ANCA-positive cases. Hemorrhagic complications in pauci-immune crescentic GN are common, but massive gastrointestinal bleeding or cerebral hemorrhage in ANCA-negative pauci-immune crescentic GN has rarely been reported. Herein, we report a rare case of recurrent massive intestinal bleeding that was caused by an uncontrolled vasculitis combined with ANCA-negative pauci-immune crescentic GN.
A 61-year-old Korean woman was admitted to our hospital with a 1-month history of generalized edema and foamy urine. On admission, she looked ill, and physical examination showed blood pressure 140/90 mmHg; pulse rate 134 beats/min; and temperature 37°C. Pitting edema greater than Grade III of the lower limbs was noted. Lung auscultation was normal, and no purpura was found on the skin. Initial laboratory test results showed white blood cell count 8,300/mm3; hemoglobin 10.7 g/L; platelets 277,000/mm3; blood urea nitrogen 54.5 mg/dL; serum creatinine 3.5 mg/dL; total protein 5.9 g/dL; albumin 3.0 g/dL; total calcium 8.4 g/dL; phosphorus 5.0 mg/dL; prothrombin time (international normalized ratio) 1.08; and activated partial thromboplastin time 34 seconds. Urinalysis revealed many red blood cells (RBCs) per high-power field, and protein (4+) and occult blood (2+) were also detected. The spot urine protein-to-creatinine ratio was 3.5 g/g.
Her renal function deteriorated rapidly over 7 days (serum creatinine concentration increased from 3.5 mg/dL to 7.0 mg/dL) and exhibited features of RPGN. Therefore, we performed a renal biopsy and other serologic tests. The serum immunoglobulin levels, including immunoglobulin (Ig)G, IgM, and IgA, and complements, including C3 and C4, were within normal ranges. Immune serologic result was negative for antinuclear antibody and antiglomerular basement membrane antibody. ANCAs also showed negative in indirect immunofluorescence and enzyme-linked immunosorbent assays.
The renal biopsy specimen showed cellular or fibrocellular crescentic formation in 92% (57/62) of glomeruli (Fig. 1). The tubules revealed focal moderate atrophy and loss with infiltration of mononuclear cells in edematous interstitium. Immunohistochemistry, using three glomeruli, demonstrated no staining for IgG, IgA, IgM, complements (C3, C1q), or fibrinogen. Electron microscopy also showed no dense deposits, with severe effacement of epithelial foot processes.
Based on these pathologic findings, she was diagnosed with ANCA-negative pauci-immune crescentic GN. The patient was treated with one cycle of steroid pulse therapy (500 mg of methylprednisolone daily, for 3 consecutive days) with monthly intravenous cyclophosphamide (400 mg), followed by prednisolone (50 mg/d).
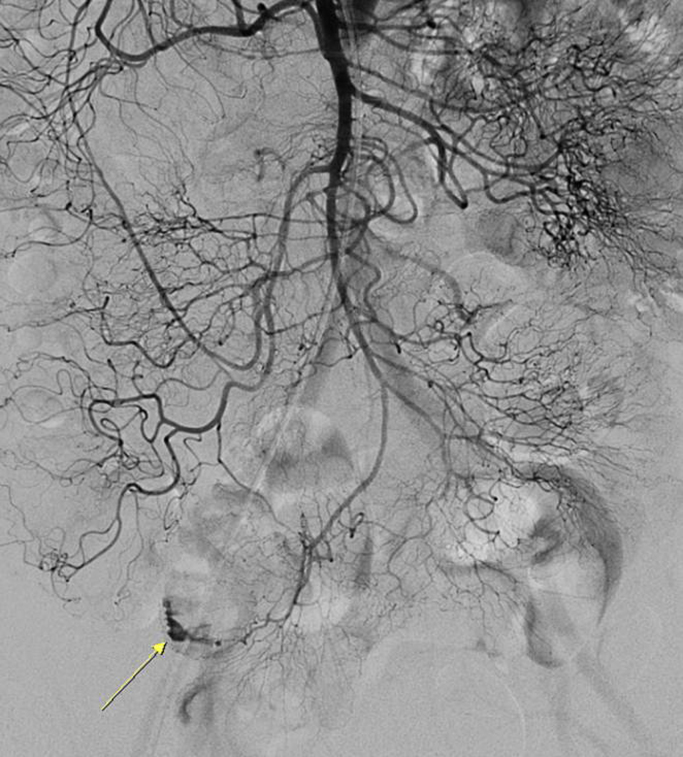
On the 15th hospital day, massive hematochezia occurred, and hemoglobin levels decreased from 9.2 mg/dL to 6.4 g/dL within 24 hours (Fig. 2). Initial resuscitation was conducted using a crystalloid solution and packed RBC transfusion. Although emergency gastroscopy and colonoscopy were performed, the bleeding focus was not detected, but a large amount of old blood was observed in the terminal ileum. After 2 days, a colonoscopy was performed again, but abnormal findings associated with bleeding were not detected. Then, a capsule endoscopy was attempted to determine if the bleeding focus was located in the small bowel. However, owing to a slow small bowel transit time, the entire small bowel could not be observed because the capsule did not reach the mid-to-distal ileum. On the 22nd hospital day, recurrent massive hematochezia occurred. Abdominal computed tomography angiography showed active arterial bleeding on the ileal loop, and selective superior mesenteric arteriography revealed an active contrast extravasation in a branch of the distal ileal artery (Fig. 3). We selectively performed embolization with a permanent embolic agent (n-butyl cyanoacrylate; 0.5 mL tissue adhesive, 1.5 mL ethionidized oil mixture), and the bleeding stopped.
Despite successful embolization, massive hematochezia occurred again after 2 days. Her hemoglobin level was 6.5 g/dL, and platelet count was 40,000/mm3. The patient received two units of packed RBCs and five units of platelet concentrate. Emergency colonoscopy showed multiple segmental ulcerations on the terminal ileum, which was consistent with ischemic damage due to embolization of the distal ileal artery (Fig. 4). The patient underwent hemodialysis because of intractable metabolic acidosis, hyperkalemia, and fluid overload. Anemia associated with schistocytes and transfusion-refractory consumptive thrombocytopenia was also present, consistent with microangiopathic hemolytic anemia (MAHA). Levels of hemostatic markers showed prothrombin time (international normalized ratio) 1.00; activated partial thromboplastin time 33 seconds; fibrinogen 399 mg/dL; and fibrin degradation product <5.0 µg/mL. We ruled out disseminated intravascular coagulation using the International Society on Thrombosis and Hemostasis disseminated intravascular coagulation scoring system.
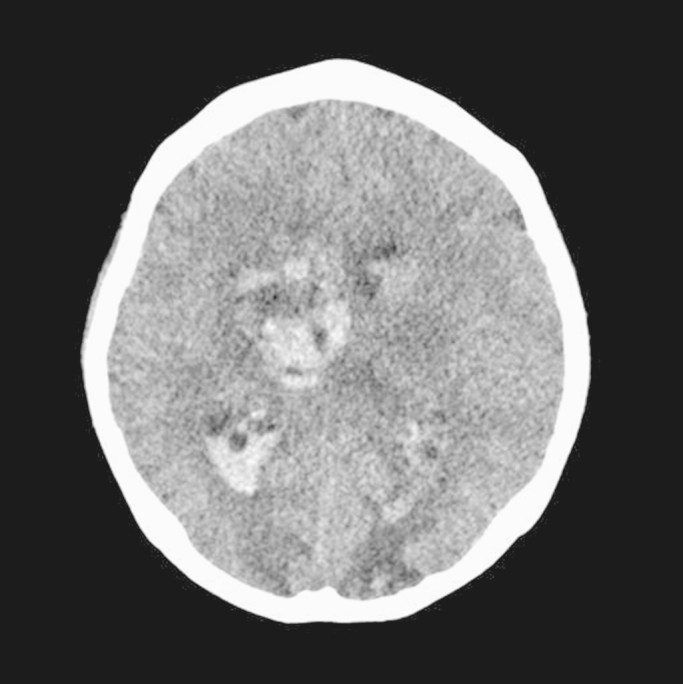
On the 45th hospital day, she suddenly lost consciousness. Brain computed tomography showed intracerebral hemorrhage (Fig. 5). The patient׳s neurologic status had worsened to a stupor and then, unfortunately, she passed away. The final diagnosis was ANCA-negative pauci-immune GN with a rare presentation of massive gastrointestinal bleeding and intracerebral hemorrhage.
Pauci-immune crescentic GN is part of systemic small-vessel vasculitis and usually associated with the presence of ANCAs directed against myeloperoxidase or proteinase 3. Although the pathogenesis of ANCA in vasculitis is not fully understood, ANCA is known to activate neutrophils by different mechanisms, such as direct Fab02 binding to ANCA antigens on leukocyte surfaces or Fc receptor engagement by ANCA immune complexes, leading to apoptosis and necrosis of neutrophil and endothelial cells [3]. However, in ANCA-negative small-vessel vasculitis, neutrophil infiltration of the pathologic lesion is reportedly related with other unidentified autoantibodies or T-cell–dependent mechanisms [4].
Despite limited information about ANCA-negative pauci-immune crescentic GN, ANCA-negative patients reportedly show severe renal manifestations, such as nephrotic-range proteinuria, poorer renal outcomes, and less extrarenal involvement such as fever, arthralgia, skin rash, abdominal pain, or mononeuritis multiplex, compared with ANCA-positive patients [5–7]. Similarly, our case also showed nephrotic-range proteinuria of 3.5 g/d and diffuse cellular crescentic formations in >90% of glomeruli on renal biopsy. Initially, extrarenal manifestations were not observed.
Our patient had no evidence of asthma, eosinophilia, or granuloma of the upper respiratory tract. Based on the 2012 International Chapel Hill Consensus criteria, we were able to diagnose MPA and exclude granulomatosis with polyangiitis and eosinophilic granulomatosis with polyangiitis from the possible systemic small-vessel vasculitis conditions. Hemorrhagic complications in patients with pauci-immune crescentic GN are common [8]. Small-vessel vasculitis can cause local or diffuse pathologic changes in the gastrointestinal tract, including ulcers, bowel wall edema, hemorrhage, paralytic ileus, ischemia, obstruction, and perforation [9–12]. Although necrotizing GN and pulmonary capillaritis are very common in MPA, several cases with gastrointestinal bleeding have been reported. Interestingly, a Japanese case diagnosed with MPA and pauci-immune crescentic GN showed active bleeding of the superior pancreaticoduodenal artery and iliac branch of the superior mesenteric artery [13]. The characteristics of this case were very similar to those of the present case: old age, absence of circulating ANCA, massive active bleeding, and absence of small artery involvement on kidney biopsy. However, our case showed a poor clinical course and a feature of MAHA. Thrombotic microangiopathy is characterized by platelet aggregation in systemic circulation, thrombocytopenia, and mechanical hemolysis. Although coexistence of thrombotic microangiopathy and pauci-immune crescentic GN is rare, pauci-immune crescentic GN could trigger thrombotic microangiopathy through endothelial damage by proinflammatory mediators [14]. Secondary ischemic multiple ulcerations after embolization, thrombocytopenia caused by consumption of platelets due to recurrent bleeding, and MAHA contribute to poor outcomes.
Initiation of conventional treatment for pauci-immune crescentic GN with or without ANCA consists of intravenous methylprednisolone pulse therapy followed by progressive reduction of oral prednisolone (initially 1 mg/kg/24 h) and cyclophosphamide, either intravenously at a dose of 0.5–1 g/m2 or orally at a dose of 2–3 mg/kg/24 h [15]. Our patient received conventional treatment according to this schedule, but massive hematochezia occurred 15 days later. In life- or organ-threatening conditions, such as lung hemorrhage or severe ANCA-related renal vasculitis, plasma exchange may be recommended. In cases with gastrointestinal bleeding, widely accepted treatment methods are lacking. Therefore, our patient only received embolization without plasmapheresis or other immunosuppressants, based on previous reports. The causes of intracerebral bleeding in our patient were not clear. Although there was a high possibility of the hemorrhagic complications of systemic vasculitis, brain magnetic resonance angiography was not performed. Various factors that could increase bleeding tendency, such as a uremic condition, low platelet count, or frequent transfusions, may contribute to intracerebral bleeding.
In summary, to the best of our knowledge, there are only a few reports regarding ANCA-negative pauci-immune crescentic GN with massive gastrointestinal bleeding. Therefore, the present case is interesting and informative, reporting recurrent massive intestinal bleeding and fatal intracerebral bleeding as a serious hemorrhagic complication of ANCA-negative pauci-immune GN.
References
2. Kerr P., Chadban S., Atkins R.. Rapidly progressive glomerulonephritis. In: Schrier R.W., Diseases of the kidney and urinary tract. Vol 11:2001. Lippincott Williams and Wilkins,; Philadelphia: p. 1665–1690.
3. Jennette J.C., Falk R.J.. The pathology of vasculitis involving the kidney. Am J Kidney Dis 124:1994;130–141.
4. Chen M., Yu F., Wang S.X., Zou W.Z., Zhao M.H., Wang H.Y.. Antineutrophil cytoplasmic autoantibody-negative pauci-immune crescentic glomerulonephritis. J Am Soc Nephrol 18:2007;599–605.


5. Hung P.H., Chiu Y.L., Lin W.C., Chiang W.C., Chen Y.M., Lin S.L., Wu K.D., Tsai T.J.. Poor renal outcome of antineutrophil cytoplasmic antibody negative pauci-immune glomerulonephritis in Taiwanese. J Formos Med Assoc 105:2006;804–812.


6. Eisenberger U., Fakhouri F., Vanhille P., Beaufils H., Mahr A., Guillevin L., Lesavre P., Noel L.H.. ANCA-negative pauci-immune renal vasculitis: histology and outcome. Nephrol Dial Transplant 20:2005;1392–1399.


7. Kumar K.S., Ramakrishnan M., Sah A.K., Gowtham S., Ajeshkumar R.N.. ANCA negative pauci-immune glomerulonephritis with systemic involvement. Indian J Nephrol 20:2001;43–47.

9. Hokama A., Kishimoto K., Ihama Y., Kobashigawa C., Nakamoto M., Hirata T., Kinjo N., Higa F., Teteyama M., Kinjo F., Iseki K., Kato S., Fujita J.. Endoscopic and radiographic features of gastrointestinal involvement in vasculitis. World J Gastrointest Endosc 4:2012;50–56.



10. Deniz K., Ozseker H.S., Balas S., Akpynar E., Sokmensuer C.. Intestinal involvement in Wegener’s granulomatosis. J Gastrointestin Liver Dis 16:2007;329–331.

11. Suzuki T., Matsushima M., Arase Y., Fujisawa M., Okita I., Igarashi M., Koike J., Mine T.. Double-ballon endoscopy-diagnosed multiple small intestinal ulcers in a Churg-Strauss syndrome patient. World J Gastrointest Endosc 4:2012;194–196.


12. Siebig S., Tarne I.H., Wrede C.E., Scholmerich J., Ladner U.M., Fleck M.. A case of recurrent abdominal bleeding due to polyarteritis nodosa despite immunosuppressive therapy. Scand J Rheumatol 36:2007;486–488.


13. Ueda S., Matsumoto M., Ahn T., Adachi S., Oku K., Takagi M., Fukui H., Yoshikawa M.. Microscopic polyangiitis complicated with massive intestinal bleeding. J Gastroenterol 36:2001;264–270.



Figure 1
Renal biopsy findings. (A) Histologic examination of a renal biopsy specimen reveals that the glomerulus has a severe crescent formation. (B) There are loop necrosis and fibrin deposition in the glomerular tufts. The tubules reveal focal moderate atrophy and loss with infiltration of mononuclear cells in edematous interstitium. Blood vessels are unremarkable. [Periodic acid-Schiff stain: (A) 400× and (B) 200×].

Figure 2
Clinical course of the patient. The black line represents hemoglobin levels (g/dL), whereas the gray line represents serum creatinine levels (mg/dL) over time. IV, intravenous.
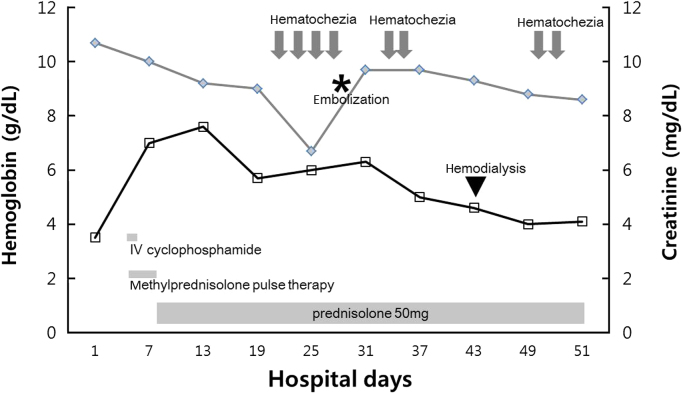
Figure 3
Superior mesenteric angiography of the patient. Selective superior mesenteric arteriography reveals an active contrast extravasation in a branch of the distal ileal artery (arrow).
- TOOLS
-
METRICS

-
- 1 Crossref
- 0 Scopus
- 1,854 View
- 12 Download
- Related articles




 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















