| Kidney Res Clin Pract > Volume 36(2); 2017 > Article |
|
Abstract
The pathophysiology of hypertension, which affects over 1 billion individuals worldwide, involves the integration of the actions of multiple organ systems, including the kidney. The kidney, which governs sodium excretion via several mechanisms including pressure natriuresis and the actions of renal sodium transporters, is central to long term blood pressure regulation and the salt sensitivity of blood pressure. The impact of renal sodium handling and the salt sensitivity of blood pressure in health and hypertension is a critical public health issue owing to the excess of dietary salt consumed globally and the significant percentage of the global population exhibiting salt sensitivity. This review highlights recent advances that have provided new insight into the renal handling of sodium and the salt sensitivity of blood pressure, with a focus on genetic, inflammatory, dietary, sympathetic nervous system and oxidative stress mechanisms that influence renal sodium excretion. Increased understanding of the multiple integrated mechanisms that regulate the renal handling of sodium and the salt sensitivity of blood pressure has the potential to identify novel therapeutic targets and refine dietary guidelines designed to treat and prevent hypertension.
Hypertension is the leading global non-communicable cause of mortality and number one risk factor for disability-adjusted life years [1–8]. Further, hypertension is the single largest risk factor for multiple co-morbidities including stroke, myocardial infarction and chronic kidney disease [6,9–12] and is projected to be the principal global cause of death and disability by 2020 [1]. The development of hypertension involves the integration of multiple regulatory systems and as a complex, multifactorial disease, our understanding of the pathogenesis remains elusive. The salt-sensitivity of blood pressure, defined as an exaggerated pressor response to dietary salt intake, affects approximately 50% of hypertensive patients and 25% of normotensive adults [13–17] and profoundly increases the risk of hypertension [13,14,18–20]. The impact of dietary salt intake on blood pressure regulation and the pathogenesis of hypertension is a global public health issue as the average global salt intake of approximately 4 g/day [21–25] significantly exceeds the World Health Organization recommendation of 2 g/day [14]. Critically, despite modifications of the classical renal centric “pressure-natriuresis hypothesis of blood pressure control” [26–28] to include genetic, sympathetic nervous system and renal mechanisms [29–34] our understanding of the role of renal sodium handling in blood pressure regulation is continually expanding. This is highlighted by a recent proposal that neurohumoral control of renal excretory function (i.e., natriuresis) represents the first line of defense against increased salt intake, followed by neurohumoral control of resistance vessels with pressure natriuresis representing a final third line of defense [35]. Therefore, given the central role of renal sodium handling in determining the salt sensitivity of blood pressure increased understanding of the mechanisms influencing renal sodium reabsorption has the potential to identify new therapeutic targets and treatment paradigms for salt-sensitive hypertension. This review is designed to highlight recent advancements in our understanding of the kidney in the etiology and pathogenesis of salt sensitive hypertension.
The influence of genetics on blood pressure regulation was first modeled in the ‘mosaic theory of hypertension’ [36] and currently hypertension is currently widely acknowledged as a complex polygenic disease state. Over the last several decades approximately 25 rare mutations and over 50 single nucleotide polymorphisms (SNPs) that impact blood pressure have been identified and were reviewed in detail by Padmanabhan et al [32] and presented in an update of the Paige mosaic model of blood pressure regulation. However, given that the majority of genome-wide association studies (GWAS) are not conducted in identified salt-sensitive versus resistant populations the identification of specific genes or markers of the salt-sensitivity of blood pressure has been challenging. Pertinent to this focused review has been the identification of SNPs in the SLC4A5 gene, which encodes the sodium-bicarbonate cotransporter, that correlated with blood pressure in non GWAS studies conducted in 2 separate populations that linked a SNPs to a causal gene (SLC4A5) and the salt-sensitivity of blood pressure [15]. Additionally, recent studies have linked SNP’s in NEDD4L [37] and SNPS located near GNAS, ZNF831 and EDN3 [38] with modulation of the responses to thiazide diuretics—findings which may have direct implications for the personalized use of this class of diuretics in antihypertensive therapy. A recent elegant study provided experimental validation and a mechanistic link between a GWAS identified gene (SH2B3) and renal inflammation in salt-sensitive hypertension. In this study a causal role of SH2B3 was delineated in the pathophysiology of Dahl salt-sensitive (DSS) hypertension. Utilizing zinc-finger nuclease technology Rudemiller et al [39] mutated the SH2B3 gene and observed attenuation of DSS rat hypertension and renal inflammation highlighting the role of this gene in the pathology of salt sensitive hypertension (Table 1). One of the patient cohorts that has been integral in the interrogation of the genetics of salt-sensitivity is the Genetic Epidemiology Network of Salt-Sensitivity Study (GenSalt). This study continues to reveal insights and a recent GWAS study of GenSalt data, confirmed by secondary analysis in the Chinese Multi-Ethnic Study of Atherosclerosis cohort, has provided 8 novel blood pressure loci that are associated with the salt-sensitivity of blood pressure [40].
Three recent publications have provided new evidence of the genetic variations associated with blood pressure. Meta-analysis of 128,272 SNPs in a total population of 432,415 adults including European, Asian and African ancestry has revealed the presence of 66 genome-wide significant blood-pressure associated loci, of which 17 are new [41]. These loci did not strongly associate with a renal target organ effect—suggesting this study did not identify a single dominant locus associated with a predominant negative impact on renal effect. A companion study that conducted the largest ever trans-ancestry meta-analysis of approximately 350,000 subjects identified 30 new blood pressure or hypertension associated loci in the general population [42]—significantly increasing our insight into the impact of genetic on blood pressure variation and potentially providing new therapeutic targets. The final study published by this team of researchers discovered a strong association between cardiometabolic risk and hypertension and identified single-variant associations at 31 new loci using meta-analysis of exome-centric single-variant and gene-based tests for association to blood pressure [43]. Of particular relevance to this review was the association of the aggregation of rare and low-frequency variants in NPR1. NPR1 encodes for atrial and B-type natriuretic peptide that have well defined roles in sodium, volume and blood pressure regulation. However, the functional significance of the low-frequency SNP rs35479618 in NPR1 remains to be established in the context of salt-sensitive hypertension [43]. A caveat to these three large scale genetic studies, in the context of the present review, is the lack of identification of the salt-sensitivity of blood pressure in the tested subjects. Despite these detailed recent advances that expand the number of genetic variants that are associated with blood pressure regulation, most notably in the collection of papers published in Nature Genetics [41–43], the major challenge remains to translate these genetic findings of SNPs into causal gene based molecular mechanisms that can be targeted to expand our ability to treat hypertension.
Studies from the past two decades have demonstrated that inflammatory cytokines are elevated in hypertensive human subjects, and elevated cytokine levels predict the risk of hypertension in normotensive patients. Although the role of inflammation in the salt sensitivity of blood pressure in humans has not been investigated, multiple animal models of salt-sensitive hypertension are characterized by renal inflammation. The role of infiltrating immune cells in the pathogenesis of salt-sensitive hypertension is highlighted by two recent animal studies. In the DSS rat, depletion of Rag1, a protein critical to the maturation of T and B lymphocytes, reduced the number of circulating T and B lymphocytes in the setting of a normal salt diet. Emphasizing the role of renal immune cell infiltration in response to salt intake, a high salt diet evoked renal T cell infiltration and increased blood pressure in control DSS rats. In contrast, T cell infiltration was reduced and hypertension was attenuated in Rag1-null rats [44]. Further supporting a role for renal immune cell accumulation in the pathophysiology of salt-sensitive hypertension, suppression of T and B lymphocyte proliferation using mycophenolate mofetil blunted dietary sodium-evoked increases in blood pressure and renal macrophage and lymphocyte infiltration and preserved pressure natriuresis in the classical L-NAME model of sodium evoked hypertension in Wistar rats [45].
The study by Franco et al in 2013 [45] directly suggests that renal immune cell infiltration may promote hypertension through alterations in renal sodium handling. Extending and supporting this hypothesis, several studies have delineated mechanistic roles for inflammatory cytokines in the regulation of renal sodium transport. In the context of angiotensin-II (Ang-II)-induced hypertension, both blood pressure and renal sodium retention are dramatically attenuated in mice lacking interferon-γ (IFN-γ−/−) or interleukin 17A (IL-17A−/−). Significantly, Ang-II-mediated increases in the total and phosphorylated forms of the sodium chloride cotransporter (NCC), but not the epithelial sodium chloride transporter (ENaC) are abolished versus attenuated in IFN-γ−/− and IL-17A−/− mice respectively, and the expression of proximal sodium transporters, including the sodium hydrogen exchanger (NHE3) and sodium potassium transporter (NaPi2), are reduced during Ang-II infusion in IFN-γ−/− and IL-17A−/− mice. These data suggest that both of these cytokines contribute to Ang-II hypertension by promoting sodium retention in the distal and proximal nephron [46]. Building upon this body of work, it has been demonstrated that IL-17A influences renal sodium transporter expression and activity in Ang-II hypertension via an SGK1-NEDD4-2 pathway [47]. Highlighting the multitude of cytokines potentially involved in regulating renal sodium transport, Ang-II-induced hypertension and sodium retention are attenuated in mice lacking the IL-1 receptor by a renal macrophage nitric oxide (NO) dependent mechanism that prevents NO-mediated suppression of NKCC2 activity [48]. A direct role for IL-1 in salt-sensitive hypertension is supported by evidence that dietary salt evokes hypertension, NLRP3 inflammasome activation, and caspase-1-mediated cleavage of IL-1β to its mature form in DSS rats. In this established rat model, infusion of a caspase-1 inhibitor into the renal medulla abolished dietary-sodium evoked increases in mature IL-1β levels and attenuated the development of salt-sensitive hypertension [49]. Further, in the DSS rat phenotype, but not in salt-resistant congenic SS.13BN26 rats, dietary salt-evoked hypertension is accompanied by increased tumor necrosis factor (TNF)-α mRNA and protein expression, and renal interstitial infusion of a TNF-α inhibitor, Etanercept, attenuated salt-sensitive hypertension [50]. These mechanistic data, generated in multiple animal models and summarized in Table 2, suggest that renal immune cell infiltration and inflammatory cytokines promote salt-sensitive hypertension by influencing renal sodium handling. Importantly, suppression of immune cell infiltration and renal inflammation promotes sodium homeostasis and normotension and may prove to be a valuable approach to the treatment of salt-sensitive hypertension in human subjects.
Dietary potassium intake has been linked with blood pressure regulation. In particular, clinical data reveals our diet is generally low in potassium and that these associates with elevated blood pressure and salt-sensitive hypertension [51], and the renal handling of potassium and sodium are intrinsically related [52]. Of particular relevance to the salt-sensitivity of blood pressure, a number of recent studies have reported that dietary potassium levels impact sodium reabsorption by modulating the activity of the NCC and ENaC in the distal nephron.
Acute potassium supplementation via oral gavage, intravenous infusion, or ingestion of a potassium-enriched meal rapidly reduces the phosphorylation and activation of the NCC, and these changes are accompanied by significant increases in natriuresis in several mouse strains and in Sprague-Dawley rats [53,54]. Additionally, potassium-evoked natriuresis is blunted in NCC-deficient mice (Sorensen et al [53]). Collectively, these data suggest a critical role of the NCC in mediating the physiological responses (i.e., natriuresis) response to acute increases in total body potassium.
Recent animal studies have provided new insight into the mechanistic links between dietary potassium and salt intake and blood pressure regulation via the NCC. In these studies, a low potassium diet blunted natriuresis and reduced dietary salt-evoked NCC suppression in mice. Further, a low potassium diet was associated with salt sensitivity of blood pressure in wild-type but not NCC-deficient mice, indicating a direct role of the NCC in the effect of dietary potassium on the salt sensitivity of blood pressure [55]. Significantly, expression irrespective of dietary sodium intake a low potassium diet resulted in increased expression and phosphorylation of with-no-lysine kinase (WNK) 4 and increased phosphorylation of SPAK, and OxSR1 [55]. These data suggest that potassium intake directly modulates NCC activity through actions upon its network of regulatory kinases. Elegant cell culture experiments by the same group have demonstrated that the distal convoluted tubule cell is hyperpolarized in the setting of low extracellular potassium, resulting in a decrease in intracellular chloride that activates the NCC via a predominant WNK4–SPAK pathway [55,56]. Despite these novel findings, it is possible that alternative pathways are involved in potassium-mediated regulation of the NCC as potassium-evoked NCC activation is maintained in WNK4 and SPAK knockout mice [57]. These studies have translational relevance, as evidenced by two human studies that have confirmed that a low potassium diet increases the abundance of pNCC in urinary exosomes collected from patients [55,58]. Dietary potassium intake has been recently demonstrated to impact the influence of Ang-II on renal sodium transporter expression in Sprague-Dawley rats. Chronic dietary potassium supplementation did not reduce Ang-II hypertension but attenuated Ang-II-induced upregulation of the NCC but not ENaC [59] suggesting differential actions of potassium on NCC vs. ENaC. The hypothesis of ENaC activation following potassium-induced suppression of sodium retention at the NCC is supported by evidence from studies conducted in mice during acute potassium supplementation [53]. The differential effects of potassium on the NCC and ENaC were highlighted by studies that demonstrated NCC upregulation and activation during low potassium intake and ENaC upregulation during high potassium intake in mice. Importantly, a low potassium-evoked salt sensitivity of blood pressure was abolished by hydrochlorothiazide in these studies [60].
Increasing dietary fructose intake in the typical western diet has mirrored the increasing prevalence of hypertension, and dietary fructose intake is associated with blood pressure in multiple animal studies [61]. In animal models, fructose promotes sodium reabsorption in the kidney, raising the possibility that fructose has a role in the salt-sensitivity of blood pressure. Two recent studies suggest that the mechanism by which fructose promotes salt sensitivity involves the increased activity of the NHE3 in the proximal nephron. Stationary micro-infusion of fructose into the proximal tubules of Wistar rats caused a dose-dependent increase in NHE3 activity, and it was demonstrated both in vivo and in vitro that fructose causes phosphorylation and activation of NHE3 via downregulation of a PKA signaling pathway [62]. In separate studies dietary fructose supplementation prior to and during increased dietary salt intake evokes the development of salt-sensitive hypertension in the salt resistant Sprague-Dawley rat [63]. Companion ex vivo studies of isolated and perfused proximal tubules suggest that 1) fructose stimulates NHE3 activity in a PKC-dependent manner and, 2) that Ang-II mediated-stimulation of NHE3 activity is enhanced by fructose [63]. Together, these studies suggest that a typical Western diet, which features low potassium intake and high fructose and sodium intake, promotes the prevalence salt-sensitive hypertension by modulating renal sodium transporter activity and expression to drive renal sodium retention (Table 3).
NO and reactive oxygen species (ROS) reciprocally alter pressure-natriuresis, and a shift in redox balance in the kidney has been implicated in human hypertension and in multiple animal models of hypertension [64]. Highlighting the importance of NO production in the maintenance of sodium balance and normotension, collecting duct-specific knockout of all isoforms of nitric oxide synthase 1 (NOS1) in mice causes sodium retention and salt-sensitive hypertension [65]. While NOS1α knockout mice do not exhibit hypertension or salt sensitivity of blood pressure, pharmacological inhibition of NOS1β in both wild-type and NOS1α knockout mice induces salt-sensitive hypertension and blunts the natriuretic and diuretic responses to an acute volume expansion, indicating a specific role for NOS1β in natriuresis and salt sensitive hypertension [66]. Recent studies indicate that NO influences the salt sensitivity of blood pressure through several independent mechanisms. Building upon an established body of literature demonstrating that NO regulates renal sodium transporter activity [67], two studies have reported that NO production is impaired in Ang-II hypertension [68], and that exogenous NO restores endothelin 1-evoked inhibition of NKCC2 activity in this model [69]. Beyond modulation of renal sodium transporters dietary salt-evoked upregulation of NOS1β in the macula densa reduces tubuloglomerular feedback responsiveness to promote sodium excretion. Significantly, macula densa-specific knockout of NOS1β results in salt-sensitive hypertension [70], indicating that NO may also influence salt sensitivity by modulating renal hemodynamic auto-regulation.
Beyond direct effects of NO renal oxidative stress and inflammation accompany salt-sensitive hypertension in mice deficient in manganese superoxide dismutase [71], and renal levels of ROS hydrogen peroxide are elevated in DSS rats during high salt intake [72]. Elegant in vivo studies have shown null mutation of the p67phox subunit of NADPH oxidase, a major source of ROS, attenuates hypertension in the DSS rat, and that dietary salt intake evokes a reduction in renal medullary blood flow and glomerular filtration rate that is blunted in p67phox-null mice [73]. Together, these studies suggest that elevated renal ROS production may contribute to salt sensitive hypertension by reducing sodium delivery and filtration in the kidney, thereby promoting sodium retention. Surprisingly, dietary salt-evoked generation of ROS by NADPH oxidase blunts myogenic autoregulation of renal blood flow and glomerular filtration rate in the salt-resistant Sprague-Dawley rat. These apparently contradictory findings raise the intriguing possibility that ROS may play an important physiological role in promoting renal sodium excretion and the maintenance of salt resistance [74].
It has recently been reported in a high profile study that salt-sensitive hypertension is driven by increased renal sympathetic nerve release of norepinephrine (NE) which evokes NCC up regulation through a WNK4-β2 adrenoceptor pathway [75]. However, the lack of a reproducible role for β2-adrenoceptors on NCC regulation in vivo in mice [76] and evidence of 1) an acute synergistic role of α- and β-adrenoceptors on NCC expression [77], 2) a key role of WNK1 in mediating NCC activation [78], and 3) lack of increased NCC expression and activity in rats during long term NE infusion [79] challenge this hypothesis. Owing to this ongoing controversy of the direct effect of sympathetic outflow, which is increased in several forms of hypertension, the impact of systemic and local release of NE on the regulation, expression and activity of the NCC requires further investigation.
The impact of the renal sympathetic nerves in the long-term regulation of blood pressure, via mechanisms that impact renal sodium reabsorption has been established for several decades. However, these classical studies, and recent human renal nerve ablation trials which demonstrated significant reductions in blood pressure in small highly controlled studies and no effect in large scale multi-center blinded trial [80–82], removed the influence of both afferent (from kidney) and efferent (to kidney) renal nerves. To address the individual actions of the afferent renal nerves a method of selective ablation of the renal afferent nerves, utilizing periaxonal capsaicin treatment has been developed in rats [83]. This technique selectively disrupts the afferent but not efferent renal nerves to provide a new tool with which to examine the contribution of the afferent renal nerves to hypertension. Removal of the afferent renal nerves prior to graded chronic increases in dietary sodium intake in the Sprague-Dawley rat suggest that the renal afferent nerves are not essential to maintain sodium homeostasis and blood pressure in this paradigm. In the classical rat model of salt sensitive hypertension, the DSS rat, recent studies show that bilateral renal denervation (removing both efferent and afferent nerves) in hypertensive animals reduces blood pressure [84] and that this effect is observed following short and long-term hypertension [85]. Further, this effect is efferent nerve mediated as selective afferent nerve ablation did not alter blood pressure. In contrast, the renal afferent nerves play a role in the full development of deoxycorticosterone acetate-salt hypertension, with afferent nerve ablation and bilateral renal denervation attenuating the magnitude of hypertension by approximately 50% [86]. However, in these studies only removal of the efferent nerves prevented a renal inflammatory response. This finding of a role of the efferent, but not afferent renal nerves in renal inflammation is supported by recent evidence that bilateral renal denervation, but not selective removal of the afferent renal nerves, attenuates Ang-II hypertension and inflammation [87]. These data collectively suggest that the differential impact of the afferent versus efferent nerves on blood pressure regulation may depend on the underlying cause of hypertension and has implications for the application of renal nerve ablation in humans.
Over the last several years, our understanding of the mechanisms that influence the renal handling of sodium and the salt sensitivity of blood pressure has dramatically increased through novel discoveries in both the basic science and genetic based clinical arenas. These findings have solidified the role of kidney as a predominant mechanism regulating blood pressure via the modulation of renal sodium excretion in multiple hypertensive disease states. The advances outlined in this review and summarized in Fig. 1 have revealed an increasingly important role of the influence of inflammatory pathways and dietary composition on renal sodium handling. Further, the recent discoveries of multiple new genes that influence both blood pressure and salt sensitivity provides an exciting opportunity to conduct casual mechanistic studies which have profound potential to increase our understanding of both renal function and hypertension. Recent basic science studies have highlighted a role of the renal sympathetic nerves in the regulation of both inflammation and renal sodium transporter activity—suggesting that depending on the underlying cause of hypertension renal nerve ablation may represent a suitable therapeutic approach. We expect the next few years will provide further delineation of the mechanisms influencing renal sodium reabsorption and will potentially identify cross talk between these pathways in the regulation of sodium homeostasis and blood pressure (i.e., genetic influences on inflammation that regulate renal sodium transport expression). We speculate that the ongoing identification and interrogation of mechanism based casual genetic pathways, inflammatory mediators, renal sympathetic nerve pathways and dietary influences on renal sodium handling will generate new therapeutic targets and refinements to current dietary guidelines that will have broad applications in the treatment of human hypertension.
Acknowledgments
This work was supported by NIH grants R01HL107330 and K02HL112718 and AHA 16MM32090001 to RDW.
Figure 1
Schematic representing the major recent advances in our understanding of renal sodium handling and the salt sensitivity of blood pressure.
EDN3, endothelin 3; ENaC, epithelial sodium channel; GNAS, guanine nucleotide-binding protein Gs; IFN-γ, interferon gamma; IL-17A, interleukin 17A; IL-1β, interleukin 1 beta; NADPH, nicotinamide adenine dinucleotide phosphate; NCC, sodium chloride cotransporter; NEDD4L, neural precursor cell expressed developmentally down-regulated gene 4-like; NHE3, sodium-hydrogen exchanger; NKCC2, sodium potassium chloride cotransporter; Nos1β, nitric oxide synthase 1; Rag1, recombinant activating gene 1; SH2B3, SH2B adaptor protein 3; SLC4A5, sodium bicarbonate cotransporter gene 4A5; TNFα, tumor necrosis factor alpha; ZNF831, zinc finger protein 831.
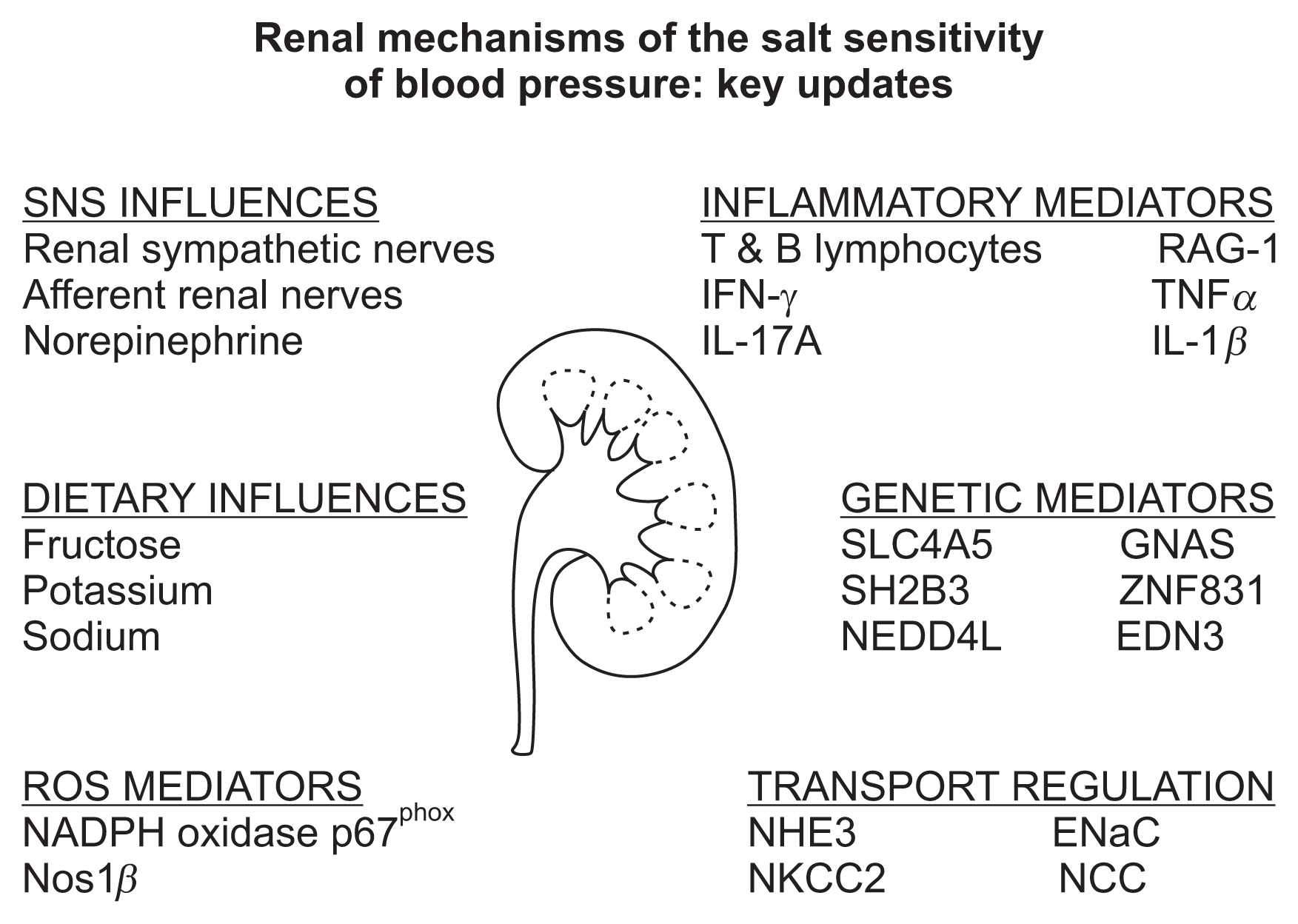
Table 1
Mechanistically defined genes and their known impact on renal sodium handling and blood pressure
| Gene | Modification | Target | Blood pressure phenotype | Reference |
|---|---|---|---|---|
| SLC4A5 | SNP | Sodium-bicarbonate exchanger | Salt-sensitive hypertension | [15] |
| NEDD4L | SNP | Unknown | Response to thiazide diuretic | [37] |
| GNAS/ZNF831/EDNS | SNP | Unknown | Response to thiazide diuretic | [38] |
| SH2BS | SNP | Renal inflammation | Salt-sensitive hypertension | [39] |
Table 2
Summary of inflammatory mediators and their impact on renal sodium transporters, sodium handling and blood pressure
| Inflammatory mediator | Target(s) | Impact on sodium handling | Blood pressure response | Reference |
|---|---|---|---|---|
| RAG-1 | T & B lymphocytes | Retention | Hypertension | [44,45] |
| IL-17A | NHE3, NaPi2, NCC, ENaC | Retention | Hypertension | [46,47] |
| IFN-γ | NHE3, NaPi2, NCC, ENaC | Retention | Hypertension | [46,47] |
| IL-1β | NKCC2, NLRP3 | Retention | Hypertension | [48] |
ENaC, epithelial sodium channel; IFN-γ, interferon gamma; IL-17A, interleukin 17A; IL-1β, interleukin 1 beta; NaPi2, sodium phosphate cotransporter 2; NCC, sodium chloride cotransporter; NHE3, sodium-hydrogen exchanger 3; NKCC2, sodium potassium chloride cotransporter; RAG-1, recombinant activating gene 1.
Table 3
Impact of dietary intake of fructose and potassium on renal sodium transporters, renal sodium handling and blood pressure
| Dietary modification | Target | Impact on renal sodium handling | Blood pressure response | Reference |
|---|---|---|---|---|
| High fructose | Increased NHE3 activity | Retention | Salt-sensitive hypertension | [61–63] |
| Acute potassium supplementation | Reduced NCC activity and phosphorylation | Excretion | ND | [53,54] |
| Acute potassium supplementation | Increased ENaC activation | Retention | ND | [53] |
| Low dietary potassium | Increased NCC activity and expression | Retention | Hypertension | [55,56,58] |
| High dietary potassium | Prevents Ang-II up regulation of NCC | Excretion | Ang-II hypertension | [59] |
References
1. Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, Farzadfar F, Stevens GA, Lim SS, Riley LM, Ezzati M. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Pressure): National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet 377:568–577. 2011;


2. Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, Amann M, Anderson HR, Andrews KG, Aryee M, Atkinson C, Bacchus LJ, Bahalim AN, Balakrishnan K, Balmes J, Barker-Collo S, Baxter A, Bell ML, Blore JD, Blyth F, Bonner C, Borges G, Bourne R, Boussinesq M, Brauer M, Brooks P, Bruce NG, Brunekreef B, Bryan-Hancock C, Bucello C, Buchbinder R, Bull F, Burnett RT, Byers TE, Calabria B, Carapetis J, Carnahan E, Chafe Z, Charlson F, Chen H, Chen JS, Cheng AT, Child JC, Cohen A, Colson KE, Cowie BC, Darby S, Darling S, Davis A, Degenhardt L, Dentener F, Des Jarlais DC, Devries K, Dherani M, Ding EL, Dorsey ER, Driscoll T, Edmond K, Ali SE, Engell RE, Erwin PJ, Fahimi S, Falder G, Farzadfar F, Ferrari A, Finucane MM, Flaxman S, Fowkes FG, Freedman G, Freeman MK, Gakidou E, Ghosh S, Giovannucci E, Gmel G, Graham K, Grainger R, Grant B, Gunnell D, Gutierrez HR, Hall W, Hoek HW, Hogan A, Hosgood HD 3rd, Hoy D, Hu H, Hubbell BJ, Hutchings SJ, Ibeanusi SE, Jacklyn GL, Jasrasaria R, Jonas JB, Kan H, Kanis JA, Kassebaum N, Kawakami N, Khang YH, Khatibzadeh S, Khoo JP, Kok C, Laden F, Lalloo R, Lan Q, Lathlean T, Leasher JL, Leigh J, Li Y, Lin JK, Lipshultz SE, London S, Lozano R, Lu Y, Mak J, Malekzadeh R, Mallinger L, Marcenes W, March L, Marks R, Martin R, McGale P, McGrath J, Mehta S, Mensah GA, Merriman TR, Micha R, Michaud C, Mishra V, Mohd Hanafiah K, Mokdad AA, Morawska L, Mozaffarian D, Murphy T, Naghavi M, Neal B, Nelson PK, Nolla JM, Norman R, Olives C, Omer SB, Orchard J, Osborne R, Ostro B, Page A, Pandey KD, Parry CD, Passmore E, Patra J, Pearce N, Pelizzari PM, Petzold M, Phillips MR, Pope D, Pope CA 3rd, Powles J, Rao M, Razavi H, Rehfuess EA, Rehm JT, Ritz B, Rivara FP, Roberts T, Robinson C, Rodriguez-Portales JA, Romieu I, Room R, Rosenfeld LC, Roy A, Rushton L, Salomon JA, Sampson U, Sanchez-Riera L, Sanman E, Sapkota A, Seedat S, Shi P, Shield K, Shivakoti R, Singh GM, Sleet DA, Smith E, Smith KR, Stapelberg NJ, Steenland K, Stöckl H, Stovner LJ, Straif K, Straney L, Thurston GD, Tran JH, Van Dingenen R, van Donkelaar A, Veerman JL, Vijayakumar L, Weintraub R, Weissman MM, White RA, Whiteford H, Wiersma ST, Wilkinson JD, Williams HC, Williams W, Wilson N, Woolf AD, Yip P, Zielinski JM, Lopez AD, Murray CJ, Ezzati M, AlMazroa MA, Memish ZA. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010 a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2224–2260. 2012;



3. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De León FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2095–2128. 2012;



4. Writing Group Members. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisa-beth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. American Heart Association Statistics Committee; Stroke Statistics Subcommittee. Executive summary: Heart disease and stroke statistics--2016 update: a report from the american heart association. Circulation 133:447–454. 2016;


5. Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Bahalim AN, Barker-Collo S, Barrero LH, Bartels DH, Basáñez MG, Baxter A, Bell ML, Benjamin EJ, Bennett D, Bernabé E, Bhalla K, Bhandari B, Bikbov B, Bin Abdulhak A, Birbeck G, Black JA, Blencowe H, Blore JD, Blyth F, Bolliger I, Bonaventure A, Boufous S, Bourne R, Boussinesq M, Braithwaite T, Brayne C, Bridgett L, Brooker S, Brooks P, Brugha TS, Bryan-Hancock C, Bucello C, Buchbinder R, Buckle G, Budke CM, Burch M, Burney P, Burstein R, Calabria B, Campbell B, Canter CE, Carabin H, Carapetis J, Carmona L, Cella C, Charlson F, Chen H, Cheng AT, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahiya M, Dahodwala N, Damsere-Derry J, Danaei G, Davis A, De Leo D, Degenhardt L, Dellavalle R, Delossantos A, Denenberg J, Derrett S, Des Jarlais DC, Dharmaratne SD, Dherani M, Diaz-Torne C, Dolk H, Dorsey ER, Driscoll T, Duber H, Ebel B, Edmond K, Elbaz A, Ali SE, Erskine H, Erwin PJ, Espindola P, Ewoigbokhan SE, Farzadfar F, Feigin V, Felson DT, Ferrari A, Ferri CP, Fèvre EM, Finucane MM, Flaxman S, Flood L, Foreman K, Forouzanfar MH, Fowkes FG, Fransen M, Freeman MK, Gabbe BJ, Gabriel SE, Gakidou E, Ganatra HA, Garcia B, Gaspari F, Gillum RF, Gmel G, Gonzalez-Medina D, Gosselin R, Grainger R, Grant B, Groeger J, Guillemin F, Gunnell D, Gupta R, Haagsma J, Hagan H, Halasa YA, Hall W, Haring D, Haro JM, Harrison JE, Havmoeller R, Hay RJ, Higashi H, Hill C, Hoen B, Hoffman H, Hotez PJ, Hoy D, Huang JJ, Ibeanusi SE, Jacobsen KH, James SL, Jarvis D, Jasrasaria R, Jayaraman S, Johns N, Jonas JB, Karthikeyan G, Kassebaum N, Kawakami N, Keren A, Khoo JP, King CH, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Laden F, Lalloo R, Laslett LL, Lathlean T, Leasher JL, Lee YY, Leigh J, Levinson D, Lim SS, Limb E, Lin JK, Lipnick M, Lipshultz SE, Liu W, Loane M, Ohno SL, Lyons R, Mabweijano J, MacIntyre MF, Malekzadeh R, Mallinger L, Manivannan S, Marcenes W, March L, Margolis DJ, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGill N, McGrath J, Medina-Mora ME, Meltzer M, Mensah GA, Merriman TR, Meyer AC, Miglioli V, Miller M, Miller TR, Mitchell PB, Mock C, Mocumbi AO, Moffitt TE, Mokdad AA, Monasta L, Montico M, Moradi-Lakeh M, Moran A, Morawska L, Mori R, Murdoch ME, Mwaniki MK, Naidoo K, Nair MN, Naldi L, Narayan KM, Nelson PK, Nelson RG, Nevitt MC, Newton CR, Nolte S, Norman P, Norman R, O’Donnell M, O’Hanlon S, Olives C, Omer SB, Ortblad K, Osborne R, Ozgediz D, Page A, Pahari B, Pandian JD, Rivero AP, Patten SB, Pearce N, Padilla RP, Perez-Ruiz F, Perico N, Pesudovs K, Phillips D, Phillips MR, Pierce K, Pion S, Polanczyk GV, Polinder S, Pope CA 3rd, Popova S, Porrini E, Pourmalek F, Prince M, Pullan RL, Ramaiah KD, Ranganathan D, Razavi H, Regan M, Rehm JT, Rein DB, Remuzzi G, Richardson K, Rivara FP, Roberts T, Robinson C, De Leòn FR, Ronfani L, Room R, Rosenfeld LC, Rushton L, Sacco RL, Saha S, Sampson U, Sanchez-Riera L, Sanman E, Schwebel DC, Scott JG, Segui-Gomez M, Shahraz S, Shepard DS, Shin H, Shivakoti R, Singh D, Singh GM, Singh JA, Singleton J, Sleet DA, Sliwa K, Smith E, Smith JL, Stapelberg NJ, Steer A, Steiner T, Stolk WA, Stovner LJ, Sudfeld C, Syed S, Tamburlini G, Tavakkoli M, Taylor HR, Taylor JA, Taylor WJ, Thomas B, Thomson WM, Thurston GD, Tleyjeh IM, Tonelli M, Towbin JA, Truelsen T, Tsilimbaris MK, Ubeda C, Undurraga EA, van der Werf MJ, van Os J, Vavilala MS, Venketasubramanian N, Wang M, Wang W, Watt K, Weatherall DJ, Weinstock MA, Weintraub R, Weisskopf MG, Weissman MM, White RA, Whiteford H, Wiebe N, Wiersma ST, Wilkinson JD, Williams HC, Williams SR, Witt E, Wolfe F, Woolf AD, Wulf S, Yeh PH, Zaidi AK, Zheng ZJ, Zonies D, Lopez AD, AlMazroa MA, Memish ZA. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010 a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380:2197–2223. 2012;


6. Olsen MH, Angell SY, Asma S, Boutouyrie P, Burger D, Chirinos JA, Damasceno A, Delles C, Gimenez-Roqueplo AP, Hering D, López-Jaramillo P, Martinez F, Perkovic V, Rietzschel ER, Schillaci G, Schutte AE, Scuteri A, Sharman JE, Wachtell K, Wang JG. A call to action and a lifecourse strategy to address the global burden of raised blood pressure on current and future generations: the Lancet Commission on hypertension. Lancet 388:2665–2712. 2016;


7. GBD 2015 Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1459–1544. 2016;


8. GBD 2015 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015 a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388:1603–1658. 2016;


9. Kannel WB. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol 85:251–255. 2000;


10. Lifton RP, Gharavi AG, Geller DS. Molecular mechanisms of human hypertension. Cell 104:545–556. 2001;


11. Mortality multiple cause micro-datafiles; public-use data file and documentation. NHLBI tabulation 2011 Available at: https://www.cdc.gov/nchs/nvss/mortality_public_use_data.htm. Date accessed: 6 May 2016.
12. A global brief on hypertension 2013 Available at: http://www.who.int/cardiovascular_diseases/publications/global_brief_hypertension/en/. Date accessed: 6 May 2016.
13. Kotchen TA, Cowley AW Jr, Frohlich ED. Salt in health and disease--a delicate balance. N Engl J Med 368:1229–1237. 2013;


14. Appel LJ, Frohlich ED, Hall JE, Pearson TA, Sacco RL, Seals DR, Sacks FM, Smith SC Jr, Vafiadis DK, Van Horn LV. The importance of population-wide sodium reduction as a means to prevent cardiovascular disease and stroke: a call to action from the American Heart Association. Circulation 123:1138–1143. 2011;


15. Carey RM, Schoeffel CD, Gildea JJ, Jones JE, McGrath HE, Gordon LN, Park MJ, Sobota RS, Underwood PC, Williams J, Sun B, Raby B, Lasky-Su J, Hopkins PN, Adler GK, Williams SM, Jose PA, Felder RA. Salt sensitivity of blood pressure is associated with polymorphisms in the sodium-bicarbonate cotransporter. Hypertension 60:1359–1366. 2012;



16. Felder RA, White MJ, Williams SM, Jose PA. Diagnostic tools for hypertension and salt sensitivity testing. Curr Opin Nephrol Hypertens 22:65–76. 2013;



17. Nichols J, Elijovich F, Laffer CL. Lack of validation of a same-day outpatient protocol for determination of salt sensitivity of blood pressure. Hypertension 59:390–394. 2012;


18. He FJ, MacGregor GA. Blood pressure--importance of salt intake. Am J Hypertens 18:1258–1259. author reply 1259–1261. 2005


19. Meneton P, Jeunemaitre X, de Wardener HE, MacGregor GA. Links between dietary salt intake, renal salt handling, blood pressure, and cardiovascular diseases. Physiol Rev 85:679–715. 2005;


20. Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, Nakamura S, Inenaga T, Kimura G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 350:1734–1737. 1997;


21. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group: Global sodium consumption and death from cardiovascular causes. N Engl J Med 371:624–634. 2014;


22. O’Donnell M, Mente A, Yusuf S. Sodium intake and cardiovascular health. Circ Res 116:1046–1057. 2015;


23. Mente A, O’Donnell M, Rangarajan S, Dagenais G, Lear S, McQueen M, Diaz R, Avezum A, Lopez-Jaramillo P, Lanas F, Li W, Lu Y, Yi S, Rensheng L, Iqbal R, Mony P, Yusuf R, Yusoff K, Szuba A, Oguz A, Rosengren A, Bahonar A, Yusufali A, Schutte AE, Chifamba J, Mann JF, Anand SS, Teo K, Yusuf S. PURE, EPIDREAM and ONTARGET/TRANSCEND Investigators. Associations of urinary sodium excretion with cardiovascular events in individuals with and without hypertension: a pooled analysis of data from four studies. Lancet 388:465–475. 2016;


24. O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S. PURE Investigators. Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 371:612–623. 2014;


25. Powles J, Fahimi S, Micha R, Khatibzadeh S, Shi P, Ezzati M, Engell RE, Lim SS, Danaei G, Mozaffarian D. Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (NutriCoDE): Global, regional and national sodium intakes in 1990 and 2010: a systematic analysis of 24 h urinary sodium excretion and dietary surveys worldwide. BMJ Open 3:e0037332013;



26. Guyton AC, Coleman TG, Cowley AV Jr, Scheel KW, Manning RD Jr, Norman RA Jr. Arterial pressure regulation. Overriding dominance of the kidneys in long-term regulation and in hypertension. Am J Med 52:584–594. 1972;

27. Guyton AC. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension 19(1 Suppl):I2–I8. 1992;


28. Guyton AC. Blood pressure control--special role of the kidneys and body fluids. Science 252:1813–1816. 1991;


29. Coffman TM. The inextricable role of the kidney in hypertension. J Clin Invest 124:2341–2347. 2014;



30. DiBona GF. Interaction of stress and dietary NaCl intake in hypertension: renal neural mechanisms. Compr Physiol 3:1741–1748. 2013;


31. Ivy JR, Bailey MA. Pressure natriuresis and the renal control of arterial blood pressure. J Physiol 592:3955–3967. 2014;



32. Padmanabhan S, Caulfield M, Dominiczak AF. Genetic and molecular aspects of hypertension. Circ Res 116:937–959. 2015;


33. Montani JP, Van Vliet BN. Understanding the contribution of Guyton’s large circulatory model to long-term control of arterial pressure. Exp Physiol 94:382–388. 2009;


34. Averina VA, Othmer HG, Fink GD, Osborn JW. A mathematical model of salt-sensitive hypertension: the neurogenic hypothesis. J Physiol 593:3065–3075. 2015;


35. Evans RG, Bie P. Role of the kidney in the pathogenesis of hypertension: time for a neo-Guytonian paradigm or a paradigm shift? Am J Physiol Regul Integr Comp Physiol 310:R217–R229. 2016;


36. Frohlich ED, Dustan HP, Bumpus FM, Irvine H. Page: 1901–1991. The celebration of a leader. Hypertension 18:443–445. 1991;


37. McDonough CW, Burbage SE, Duarte JD, Gong Y, Langaee TY, Turner ST, Gums JG, Chapman AB, Bailey KR, Beitelshees AL, Boerwinkle E, Pepine CJ, Cooper-DeHoff RM, Johnson JA. Association of variants in NEDD4L with blood pressure response and adverse cardiovascular outcomes in hypertensive patients treated with thiazide diuretics. J Hypertens 31:698–704. 2013;



38. Turner ST, Boerwinkle E, O’Connell JR, Bailey KR, Gong Y, Chapman AB, McDonough CW, Beitelshees AL, Schwartz GL, Gums JG, Padmanabhan S, Hiltunen TP, Citterio L, Donner KM, Hedner T, Lanzani C, Melander O, Saarela J, Ripatti S, Wahlstrand B, Manunta P, Kontula K, Dominiczak AF, Cooper-DeHoff RM, Johnson JA. Genomic association analysis of common variants influencing antihypertensive response to hydrochlorothiazide. Hypertension 62:391–397. 2013;



39. Rudemiller NP, Lund H, Priestley JR, Endres BT, Prokop JW, Jacob HJ, Geurts AM, Cohen EP, Mattson DL. Mutation of SH2B3 (LNK), a genome-wide association study candidate for hypertension, attenuates Dahl salt-sensitive hypertension via inflammatory modulation. Hypertension 65:1111–1117. 2015;



40. Li C, He J, Chen J, Zhao J, Gu D, Hixson JE, Rao DC, Jaquish CE, Gu CC, Chen J, Huang J, Chen S, Kelly TN. Genome-wide gene-sodium interaction analyses on blood pressure: the Genetic Epidemiology Network of Salt-Sensitivity Study. Hypertension 68:348–355. 2016;



41. Ehret GB, Ferreira T, Chasman DI, Jackson AU, Schmidt EM, Johnson T, Thorleifsson G, Luan J, Donnelly LA, Kanoni S, Petersen AK, Pihur V, Strawbridge RJ, Shungin D, Hughes MF, Meirelles O, Kaakinen M, Bouatia-Naji N, Kristiansson K, Shah S, Kleber ME, Guo X, Lyytikäinen LP, Fava C, Eriksson N, Nolte IM, Magnusson PK, Salfati EL, Rallidis LS, Theusch E, Smith AJ, Folkersen L, Witkowska K, Pers TH, Joehanes R, Kim SK, Lataniotis L, Jansen R, Johnson AD, Warren H, Kim YJ, Zhao W, Wu Y, Tayo B, Bochud M. CHARGE-EchoGen Consortium; CHARGE-HF Consortium; Wellcome Trust Case Control Consortium. Absher D, Adair LS, Amin N, Arking DE, Axelsson T, Baldassarre D, Balkau B, Bandinelli S, Barnes MR, Barroso I, Bevan S, Bis JC, Bjornsdottir G, Boehnke M, Boerwinkle E, Bonnycastle LL, Boomsma DI, Bornstein SR, Brown MJ, Burnier M, Cabrera CP, Chambers JC, Chang IS, Cheng CY, Chines PS, Chung RH, Collins FS, Connell JM, Döring A, Dallongeville J, Danesh J, de Faire U, Delgado G, Dominiczak AF, Doney AS, Drenos F, Edkins S, Eicher JD, Elosua R, Enroth S, Erdmann J, Eriksson P, Esko T, Evangelou E, Evans A, Fall T, Farrall M, Felix JF, Ferrières J, Ferrucci L, Fornage M, Forrester T, Franceschini N, Franco OH, Franco-Cereceda A, Fraser RM, Ganesh SK, Gao H, Gertow K, Gianfagna F, Gigante B, Giulianini F, Goel A, Goodall AH, Goodarzi MO, Gorski M, Gräßler J, Groves CJ, Gudnason V, Gyllensten U, Hallmans G, Hartikainen AL, Hassinen M, Havulinna AS, Hayward C, Hercberg S, Herzig KH, Hicks AA, Hingorani AD, Hirschhorn JN, Hofman A, Holmen J, Holmen OL, Hottenga JJ, Howard P, Hsiung CA, Hunt SC, Ikram MA, Illig T, Iribarren C, Jensen RA, Kähönen M, Kang HM, Kathiresan S, Keating BJ, Khaw KT, Kim YK, Kim E, Kivimaki M, Klopp N, Kolovou G, Komulainen P, Kooner JS, Kosova G, Krauss RM, Kuh D, Kutalik Z, Kuusisto J, Kvaløy K, Lakka TA, Lee NR, Lee IT, Lee WJ, Levy D, Li X, Liang KW, Lin H, Lin L, Lindström J, Lobbens S, Männistö S, Müller G, Müller-Nurasyid M, Mach F, Markus HS, Marouli E, McCarthy MI, McKenzie CA, Meneton P, Menni C, Metspalu A, Mijatovic V, Moilanen L, Montasser ME, Morris AD, Morrison AC, Mulas A, Nagaraja R, Narisu N, Nikus K, O’Donnell CJ, O’Reilly PF, Ong KK, Paccaud F, Palmer CD, Parsa A, Pedersen NL, Penninx BW, Perola M, Peters A, Poulter N, Pramstaller PP, Psaty BM, Quertermous T, Rao DC, Rasheed A, Rayner NW, Renström F, Rettig R, Rice KM, Roberts R, Rose LM, Rossouw J, Samani NJ, Sanna S, Saramies J, Schunkert H, Sebert S, Sheu WH, Shin YA, Sim X, Smit JH, Smith AV, Sosa MX, Spector TD, Stančáková A, Stanton AV, Stirrups KE, Stringham HM, Sundstrom J, Swift AJ, Syvänen AC, Tai ES, Tanaka T, Tarasov KV, Teumer A, Thorsteinsdottir U, Tobin MD, Tremoli E, Uitterlinden AG, Uusitupa M, Vaez A, Vaidya D, van Duijn CM, van Iperen EP, Vasan RS4, Verwoert GC, Virtamo J, Vitart V, Voight BF, Vollenweider P, Wagner A, Wain LV, Wareham NJ, Watkins H, Weder AB, Westra HJ, Wilks R, Wilsgaard T, Wilson JF, Wong TY, Yang TP, Yao J, Yengo L, Zhang W, Zhao JH, Zhu X, Bovet P, Cooper RS, Mohlke KL, Saleheen D, Lee JY, Elliott P, Gierman HJ, Willer CJ, Franke L, Hovingh GK, Taylor KD, Dedoussis G, Sever P, Wong A, Lind L, Assimes TL, Njølstad I, Schwarz PE, Langenberg C, Snieder H, Caulfield MJ, Melander O, Laakso M, Saltevo J, Rauramaa R, Tuomilehto J, Ingelsson E, Lehtimäki T, Hveem K, Palmas W, März W, Kumari M, Salomaa V, Chen YD, Rotter JI, Froguel P, Jarvelin MR, Lakatta EG, Kuulasmaa K, Franks PW, Hamsten A, Wichmann HE, Palmer CN, Stefansson K, Ridker PM, Loos RJ, Chakravarti A, Deloukas P, Morris AP, Newton-Cheh C, Munroe PB. The genetics of blood pressure regulation and its target organs from association studies in 342,415 individuals. Nat Genet 48:1171–1184. 2016;



42. Surendran P, Drenos F, Young R, Warren H, Cook JP, Manning AK, Grarup N, Sim X, Barnes DR, Witkowska K, Staley JR, Tragante V, Tukiainen T, Yaghootkar H, Masca N, Freitag DF, Ferreira T, Giannakopoulou O, Tinker A, Harakalova M, Mihailov E, Liu C, Kraja AT, Nielsen SF, Rasheed A, Samuel M, Zhao W, Bonnycastle LL, Jackson AU, Narisu N, Swift AJ, Southam L, Marten J, Huyghe JR, Stancakova A, Fava C, Ohlsson T, Matchan A, Stirrups KE, Bork-Jensen J, Gjesing AP, Kontto J, Perola M, Shaw-Hawkins S, Havulinna AS, Zhang H, Donnelly LA, Groves CJ, Rayner NW, Neville MJ, Robertson NR, Yiorkas AM, Herzig KH, Kajantie E, Zhang W, Willems SM, Lannfelt L, Malerba G, Soranzo N, Trabetti E, Verweij N, Evangelou E, Moayyeri A, Vergnaud AC, Nelson CP, Poveda A, Varga TV, Caslake M, de Craen AJ, Trompet S, Luan J, Scott RA, Harris SE, Liewald DC, Marioni R, Menni C, Farmaki AE, Hallmans G, Renstrom F, Huffman JE, Hassinen M, Burgess S, Vasan RS, Felix JF, Consortium CH-HF, Uria-Nickelsen M, Malarstig A, Reilly DF, Hoek M, Vogt TF, Lin H, Lieb W, EchoGen C, Traylor M, Markus HS, Consortium M, Highland HM, Justice AE, Marouli E, Consortium G, Lindstrom J, Uusitupa M, Komulainen P, Lakka TA, Rauramaa R, Polasek O, Rudan I, Rolandsson O, Franks PW, Dedoussis G, Spector TD, Consortium EP-I, Jousilahti P, Mannisto S, Deary IJ, Starr JM, Langenberg C, Wareham NJ, Brown MJ, Dominiczak AF, Connell JM, Jukema JW, Sattar N, Ford I, Packard CJ, Esko T, Magi R, Metspalu A, de Boer RA, van der Meer P, van der Harst P, Lifelines Cohort S, Gambaro G, Ingelsson E, Lind L, de Bakker PI, Numans ME, Brandslund I, Christensen C, Petersen ER, Korpi-Hyovalti E, Oksa H, Chambers JC, Kooner JS, Blakemore AI, Franks S, Jarvelin MR, Husemoen LL, Linneberg A, Skaaby T, Thuesen B, Karpe F, Tuomilehto J, Doney AS, Morris AD, Palmer CN, Holmen OL, Hveem K, Willer CJ, Tuomi T, Groop L, Karajamaki A, Palotie A, Ripatti S, Salomaa V, Alam DS, Majumder AA, Di Angelantonio E, Chowdhury R, McCarthy MI, Poulter N, Stanton AV, Sever P, Amouyel P, Arveiler D, Blankenberg S, Ferrieres J, Kee F, Kuulasmaa K, Muller-Nurasyid M, Veronesi G, Virtamo J, Deloukas P. Wellcome Trust Case Control C. Elliott P. Understanding Society Scientific G. Zeggini E, Kathiresan S, Melander O, Kuusisto J, Laakso M, Padmanabhan S, Porteous DJ, Hayward C, Scotland G, Collins FS, Mohlke KL, Hansen T, Pedersen O, Boehnke M, Stringham HM, Consortium E-C, Frossard P, Newton-Cheh C, Consortium CECBP, Tobin MD, Nordestgaard BG, Consortium TDG, Go TDC, Exome BPC, Consortium CHDE, Caulfield MJ, Mahajan A, Morris AP, Tomaszewski M, Samani NJ, Saleheen D, Asselbergs FW, Lindgren CM, Danesh J, Wain LV, Butterworth AS, Howson JM, Munroe PB. Trans-ancestry meta-analyses identify rare and common variants associated with blood pressure and hypertension. Nat Genet 48:1151–1161. 2016;



43. Liu C, Kraja AT, Smith JA, Brody JA, Franceschini N, Bis JC, Rice K, Morrison AC, Lu Y, Weiss S, Guo X, Palmas W, Martin LW, Chen YI, Surendran P, Drenos F, Cook JP, Auer PL, Chu AY, Giri A, Zhao W, Jakobsdottir J, Lin LA, Stafford JM, Amin N, Mei H, Yao J, Voorman A, Consortium CHDE, Exome BPC, Go TDC, Consortium TDG, Larson MG, Grove ML, Smith AV, Hwang SJ, Chen H, Huan T, Kosova G, Stitziel NO, Kathiresan S, Samani N, Schunkert H, Deloukas P. Myocardial Infarction G. Consortia CAE, Li M, Fuchsberger C, Pattaro C, Gorski M, Consortium CK, Kooperberg C, Papanicolaou GJ, Rossouw JE, Faul JD, Kardia SL, Bouchard C, Raffel LJ, Uitterlinden AG, Franco OH, Vasan RS, O’Donnell CJ, Taylor KD, Liu K, Bottinger EP, Gottesman O, Daw EW, Giulianini F, Ganesh S, Salfati E, Harris TB, Launer LJ, Dorr M, Felix SB, Rettig R, Volzke H, Kim E, Lee WJ, Lee IT, Sheu WH, Tsosie KS, Edwards DR, Liu Y, Correa A, Weir DR, Volker U, Ridker PM, Boerwinkle E, Gudnason V, Reiner AP, van Duijn CM, Borecki IB, Edwards TL, Chakravarti A, Rotter JI, Psaty BM, Loos RJ, Fornage M, Ehret GB, Newton-Cheh C, Levy D, Chasman DI. Meta-analysis identifies common and rare variants influencing blood pressure and overlapping with metabolic trait loci. Nat Genet 48:1162–1170. 2016;



44. Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM, Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol 304:R407–R414. 2013;



45. Franco M, Tapia E, Bautista R, Pacheco U, Santamaria J, Quiroz Y, Johnson RJ, Rodriguez-Iturbe B. Impaired pressure natriuresis resulting in salt-sensitive hypertension is caused by tubulointerstitial immune cell infiltration in the kidney. Am J Physiol Renal Physiol 304:F982–F990. 2013;



46. Kamat NV, Thabet SR, Xiao L, Saleh MA, Kirabo A, Madhur MS, Delpire E, Harrison DG, McDonough AA. Renal transporter activation during angiotensin-II hypertension is blunted in interferon-γ−/− and interleukin-17A−/− mice. Hypertension 65:569–576. 2015;



47. Norlander AE, Saleh MA, Kamat NV, Ko B, Gnecco J, Zhu L, Dale BL, Iwakura Y, Hoover RS, McDonough AA, Madhur MS. Interleukin-17A regulates renal sodium transporters and renal injury in angiotensin II-induced hypertension. Hypertension 68:167–174. 2016;



48. Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD, Crowley SD. Interleukin-1 receptor activation potentiates salt re-absorption in angiotensin II-induced hypertension via the NKCC2 co-transporter in the nephron. Cell Metab 23:360–368. 2016;


49. Zhu Q, Li XX, Wang W, Hu J, Li PL, Conley S, Li N. Mesenchymal stem cell transplantation inhibited high salt-induced activation of the NLRP3 inflammasome in the renal medulla in Dahl S rats. Am J Physiol Renal Physiol 310:F621–F627. 2016;



50. Huang B, Cheng Y, Usa K, Liu Y, Baker MA, Mattson DL, He Y, Wang N, Liang M. Renal tumor necrosis factor α contributes to hypertension in Dahl salt-sensitive rats. Sci Rep 6(21960):2016.

51. Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S. PURE Investigators. Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 371:601–611. 2014;


52. Penton D, Czogalla J, Loffing J. Dietary potassium and the renal control of salt balance and blood pressure. Pflugers Arch 467:513–530. 2015;


53. Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J. Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83:811–824. 2013;


54. Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, Mc-Donough AA. Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306:F1059–F1068. 2014;



55. Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL, Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab 21:39–50. 2015;



56. Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH. Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89:127–134. 2016;



57. Castañeda-Bueno M, Cervantes-Perez LG, Rojas-Vega L, Arroyo-Garza I, Vázquez N, Moreno E, Gamba G. Modulation of NCC activity by low and high K(+) intake: insights into the signaling pathways involved. Am J Physiol Renal Physiol 306:F1507–F1519. 2014;



58. Ellison DH, Terker AS. Why your mother was right: how potassium intake reduces blood pressure. Trans Am Clin Climatol Assoc 126:46–55. 2015;


59. Veiras LC, Han J, Ralph DL, McDonough AA. Potassium supplementation prevents sodium chloride cotransporter stimulation during angiotensin II hypertension. Hypertension 68:904–912. 2016;



60. Vitzthum H, Seniuk A, Schulte LH, Müller ML, Hetz H, Ehmke H. Functional coupling of renal K+ and Na+ handling causes high blood pressure in Na+ replete mice. J Physiol 592:1139–1157. 2014;



61. Ares GR, Ortiz PA. Direct renal effects of a fructose-enriched diet: interaction with high salt intake. Am J Physiol Regul Integr Comp Physiol 309:R1078–R1081. 2015;



62. Queiroz-Leite GD, Crajoinas RO, Neri EA, Bezerra CN, Girardi AC, Rebouças NA, Malnic G. Fructose acutely stimulates NHE3 activity in kidney proximal tubule. Kidney Blood Press Res 36:320–334. 2012;


63. Cabral PD, Hong NJ, Hye Khan MA, Ortiz PA, Beierwaltes WH, Imig JD, Garvin JL. Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 63:e68–e73. 2014;


64. Cowley AW Jr, Abe M, Mori T, O’Connor PM, Ohsaki Y, Zheleznova NN. Reactive oxygen species as important determinants of medullary flow, sodium excretion, and hypertension. Am J Physiol Renal Physiol 308:F179–F197. 2015;


65. Hyndman KA, Boesen EI, Elmarakby AA, Brands MW, Huang P, Kohan DE, Pollock DM, Pollock JS. Renal collecting duct NOS1 maintains fluid-electrolyte homeostasis and blood pressure. Hypertension 62:91–98. 2013;



66. Wang X, Chandrashekar K, Wang L, Lai EY, Wei J, Zhang G, Wang S, Zhang J, Juncos LA, Liu R. Inhibition of nitric oxide synthase 1 induces salt-sensitive hypertension in nitric oxide synthase 1α knockout and wild-type mice. Hypertension 67:792–799. 2016;



67. Horita S, Nakamura M, Shirai A, Yamazaki O, Satoh N, Suzuki M, Seki G. Regulatory roles of nitric oxide and angiotensin II on renal tubular transport. World J Nephrol 3:295–301. 2014;



68. Ramseyer VD, Gonzalez-Vicente A, Carretero OA, Garvin JL. Angiotensin II-induced hypertension blunts thick ascending limb NO production by reducing NO synthase 3 expression and enhancing threonine 495 phosphorylation. Am J Physiol Renal Physiol 308:F149–F156. 2015;


69. Ramseyer VD, Ortiz PA, Carretero OA, Garvin JL. Angiotensin II-mediated hypertension impairs nitric oxide-induced NKCC2 inhibition in thick ascending limbs. Am J Physiol Renal Physiol 310:F748–F754. 2016;



70. Lu Y, Wei J, Stec DE, Roman RJ, Ge Y, Cheng L, Liu EY, Zhang J, Hansen PB, Fan F, Juncos LA, Wang L, Pollock J, Huang PL, Fu Y, Wang S, Liu R. Macula densa nitric oxide synthase 1β protects against salt-sensitive hypertension. J Am Soc Nephrol 27:2346–2356. 2016;


71. Jin K, Vaziri ND. Salt-sensitive hypertension in mitochondrial superoxide dismutase deficiency is associated with intra-renal oxidative stress and inflammation. Clin Exp Nephrol 18:445–452. 2014;


72. Palygin O, Levchenko V, Ilatovskaya DV, Pavlov TS, Ryan RP, Cowley AW Jr, Staruschenko A. Real-time electrochemical detection of ATP and H2O2 release in freshly isolated kidneys. Am J Physiol Renal Physiol 305:F134–F141. 2013;



73. Evans LC, Ryan RP, Broadway E, Skelton MM, Kurth T, Cowley AW Jr. Null mutation of the nicotinamide adenine dinucleotide phosphate-oxidase subunit p67phox protects the Dahl-S rat from salt-induced reductions in medullary blood flow and glomerular filtration rate. Hypertension 65:561–568. 2015;


74. Fellner RC, Cook AK, O’Connor PM, Zhang S, Pollock DM, Inscho EW. High-salt diet blunts renal autoregulation by a reactive oxygen species-dependent mechanism. Am J Physiol Renal Physiol 307:F33–F40. 2014;



75. Mu S, Shimosawa T, Ogura S, Wang H, Uetake Y, Kawakami-Mori F, Marumo T, Yatomi Y, Geller DS, Tanaka H, Fujita T. Epigenetic modulation of the renal β-adrenergic-WNK4 pathway in salt-sensitive hypertension. Nat Med 17:573–580. 2011;


76. Uchida S, Chiga M, Sohara E, Rai T, Sasaki S. Does a β2-adrenergic receptor-WNK4-Na-Cl co-transporter signal cascade exist in the in vivo kidney? Nat Med 18:1324–1325. author reply 1325–1327. 2012


77. Terker AS, Yang CL, McCormick JA, Meermeier NP, Rogers SL, Grossmann S, Trompf K, Delpire E, Loffing J, Ellison DH. Sympathetic stimulation of thiazide-sensitive sodium chloride cotransport in the generation of salt-sensitive hypertension. Hypertension 64:178–184. 2014;



78. Chávez-Canales M, Zhang C, Soukaseum C, Moreno E, Pacheco-Alvarez D, Vidal-Petiot E, Castañeda-Bueno M, Vázquez N, Rojas-Vega L, Meermeier NP, Rogers S, Jeunemaitre X, Yang CL, Ellison DH, Gamba G, Hadchouel J. WNK-SPAK-NCC cascade revisited: WNK1 stimulates the activity of the Na-Cl cotransporter via SPAK, an effect antagonized by WNK4. Hypertension 64:1047–1053. 2014;



79. Walsh KR, Kuwabara JT, Shim JW, Wainford RD. Norepinephrine-evoked salt-sensitive hypertension requires impaired renal sodium chloride cotransporter activity in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 310:R115–R124. 2016;


80. Bhatt DL, Kandzari DE, O’Neill WW, D’Agostino R, Flack JM, Katzen BT, Leon MB, Liu M, Mauri L, Negoita M, Cohen SA, Oparil S, Rocha-Singh K, Townsend RR, Bakris GL. SYMPLICITY HTN-3 Investigators. A controlled trial of renal denervation for resistant hypertension. N Engl J Med 370:1393–1401. 2014;


81. Krum H, Schlaich MP, Sobotka PA, Böhm M, Mahfoud F, Rocha-Singh K, Katholi R, Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet 383:622–629. 2014;


82. Esler MD, Böhm M, Sievert H, Rump CL, Schmieder RE, Krum H, Mahfoud F, Schlaich MP. Catheter-based renal denervation for treatment of patients with treatment-resistant hypertension: 36 month results from the SYMPLICITY HTN-2 randomized clinical trial. Eur Heart J 35:1752–1759. 2014;



83. Foss JD, Wainford RD, Engeland WC, Fink GD, Osborn JW. A novel method of selective ablation of afferent renal nerves by periaxonal application of capsaicin. Am J Physiol Regul Integr Comp Physiol 308:R112–R122. 2015;


84. Foss JD, Fink GD, Osborn JW. Reversal of genetic salt-sensitive hypertension by targeted sympathetic ablation. Hypertension 61:806–811. 2013;



85. Foss JD, Fink GD, Osborn JW. Differential role of afferent and efferent renal nerves in the maintenance of early- and late-phase Dahl S hypertension. Am J Physiol Regul Integr Comp Physiol 310:R262–R267. 2010;

86. Banek CT, Knuepfer MM, Foss JD, Fiege JK, Asirvatham-Jeyaraj N, Van Helden D, Shimizu Y, Osborn JW. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension 68:1415–1423. 2016;



87. Itani HA, Xiao L, Saleh MA, Wu J, Pilkinton MA, Dale BL, Barbaro NR, Foss JD, Kirabo A, Montaniel KR, Norlander AE, Chen W, Sato R, Navar LG, Mallal SA, Madhur MS, Bernstein KE, Harrison DG. CD70 exacerbates blood pressure elevation and renal damage in response to repeated hypertensive stimuli. Circ Res 118:1233–1243. 2016;



- TOOLS



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















