Introduction
Renal artery thrombosis is a rare but serious clinical problem because it frequently causes renal infarction. If perfusion of kidney is not restored in time, the involved segment or whole kidney may lose function. However, early diagnosis is not easy because clinical presentation, such as hematuria or flank pain, is not specific. Therefore, understanding the risk factors and having high suspicion are very important.
Renal artery thrombosis is mainly associated with thromboembolism from heart, atherosclerosis, fibromuscular dysplasia, or cocaine abuse [1], [2]. Renal artery thrombosis can also occur in a hypercoagulable state [3], [4], [5]. There are some reports of renal vein thrombosis associated with acute pyelonephritis [6], [7], [9], [10], [11], [8], but renal artery thrombosis related to acute pyelonephritis has not been reported yet. Herein, we describe a case of renal artery thrombosis that developed during the recovery phase of acute pyelonephritis complicated with sepsis in a patient with diabetes.
Case report
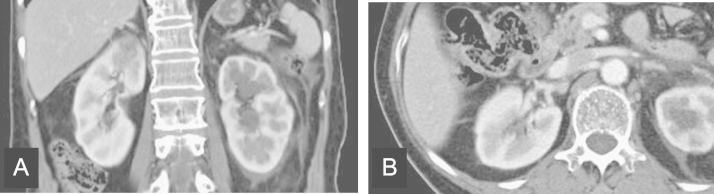
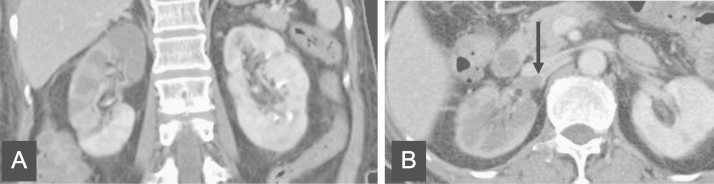
A 65-year-old woman presented to the emergency room complaining of right flank pain and fever. She has suffered from diabetes for 20 years and was using oral hypoglycemic agents. Physical examination revealed the following: blood pressure, 80/50┬ĀmmHg; temperature, 39┬Ā┬░C; pulse, 130 beats/minute; and respiration rate, 20 breaths/minute. Knocking tenderness was noted on the right flank area. Her blood test results were as follows: white blood cell (WBC) count, 23,400/╬╝L; hemoglobin, 9.7┬Āg/dL; platelet count, 97,000/╬╝L; C-reactive protein, 21.97┬Āmg/dL; blood urea nitrogen, 35.3┬Āmg/dL; creatinine (Cr), 2.21┬Āmg/dL; sodium, 142 mEq/L; potassium, 3.7 mEq/L; total protein, 4.8┬Āg/dL; albumin, 2.4┬Āg/dL; lactate dehydrogenase (LDH), 472 U/L; and HbA1c, 9.8%. Prothrombin time (PT) was found to be 17.5┬Āseconds (international normalized ratio or INR: 1.63, 46.8%) and activated partial thromboplastin time (aPTT) increased to 52.8┬Āseconds. Antithrombin III level decreased to 70.5% and fibrin degradation products (FDPs) and fibrinogen levels were elevated to 49.2┬Ā╬╝g/mL and 429.3┬Āmg/dL, respectively. Results of urinalysis revealed the following: specific gravity, 1.028; pH: 5.0; traces of protein; 10ŌĆō19 WBCs and 0ŌĆō1 red blood cells (RBCs) per high power field. An electrocardiography test showed sinus tachycardia. A KUB finding was nonspecific. To verify the cause of acute abdominal pain, a contrast-enhanced abdominal computed tomography (CT) scan was performed (Fig. 1). There was a focal wedge-shaped perfusion defect in the upper pole of right kidney, but no evidence of intravascular filling defect in renal vessels was found. Escherichia coli was isolated from both blood and urine cultures. Under the diagnosis of right acute pyelonephritis complicated with sepsis, intravenous ceftriaxone 2┬Āg per day was administered. Five days later, her mental status became drowsy, blood pressure fell to 80/60┬ĀmmHg, and fever developed. A chest X-ray showed increased haziness on both lower lung fields. Laboratory tests revealed the following: WBC count, 27,800/╬╝L; platelet count, 26,000/╬╝L; and Cr 0.8┬Āmg/dL. To rule out pneumonia, a chest CT scan was performed, which revealed passive atelectasis in both lungs, but no evidence of pneumonia. An echocardiography showed mildly decreased cardiac wall motion and left ventricular dysfunction (ejection fraction: 47%), but no evidence of thrombus in all cardiac chambers. With the impression of septic shock combined with acute heart failure, diuretics and inotropes were administered, and antibiotics were changed to piperacillin sodium 4┬Āg and tazobactam 0.5┬Āg every 8┬Āhours. Her clinical course gradually improved after changing antibiotics. On the 13th day of hospitalization, she complained of sudden onset of severe right flank pain and tenderness. Urinalysis revealed 1ŌĆō4 WBCs and 5ŌĆō9 RBCs per high power field. The level of LDH increased to 1768 U/L. An abdominal CT revealed newly developed large perfusion defect in the upper to mid portion of the right kidney and intravascular filling defect in the right renal artery (Fig. 2). To evaluate coagulation defects, additional laboratory tests were carried out which revealed the following: PT, 12.3┬Ās (INR: 1.14, 78.3%), aPTT, 23.8┬Ās; antithrombin III, 84.7%; FDP, 19.5┬Ā╬╝g/mL; fibrinogen >500┬Āmg/dL; and factor assay V, 81%. Proteins C and S were normal and lupus anticoagulant Ab was not found. On the 16th day of hospitalization, the patientŌĆÖs flank pain resolved and hematuria disappeared. A follow-up abdominal CT scan demonstrated that the perfusion defect in the right kidney and the size of right renal artery thrombus were partially regressed. The patient was discharged on oral antibiotics in a healthy condition on 22nd day.
Discussion
In this report, renal artery thrombosis was observed in the recovery phase of acute pyelonephritis and septic shock. The patient has suffered from diabetes for 20 years, but did not have other risk factors for arterial thrombosis. Although the cause of renal artery thrombosis is not fully understood in this case, several mechanisms are suggested. Arterial thrombosis can occur in the condition of endothelial damage, hypercoagulable state, kidney transplantation, or thromboembolism from heart. Mostly, it is generated in the heart with arrhythmia, valvular heart disease, or ischemic heart disease. This patient had normal sinus rhythm. There was no paroxysmal atrial fibrillation or arrhythmia in intensive care unit measurements. In addition, no cardiac abnormality or intracardiac thrombus was found in the echocardiography. There was no abnormality in protein C/S activity or factor V Leiden mutation. Therefore, it is not likely that the hypercoagulability and emboli from heart contribute to the thrombus formation. Because she has suffered from diabetes for 20 years, atherosclerosis could have contributed to arterial thrombosis formation, but no thrombus or stenosis was noted on initial abdominal CT scan.
In this report, the patient had acute pyelonephritis and sepsis-induced disseminated intravascular coagulation (DIC). Biochemical markers revealed low platelet count and prolonged PT, aPTT, and elevated fibrinogen levels, which were consistent with DIC criteria.
There were instances where an autopsy examination revealed the precipitation of fibrin thrombi in multiple organs of cases with DIC disorder [9]. While kidney was involved in 15 of the 22 cases studied (68%), most of the cases involved veins or small vessels such as arterioles. However, only one case that had Gram-negative pneumonia-associated DIC had renal artery involvement. However, there was an aneurysmal change in the renal artery, which could contribute to thrombus formation.
Sepsis is often associated with hemostatic abnormalities ranging from isolated thrombocytopenia, hypercoagulability to DIC. In sepsis-associated DIC, the key event is the systemic inflammatory response to the infectious agent. DIC is frequently associated with Gram-negative bacterial infection but it can occur with a similar incidence in Gram-positive infection. Results of several studies have shown that patients with severe infection are at increased risk of venous thrombosis and pulmonary embolism [10], [11]. Also, there were several reports on renal vein thrombosis associated with pyelonephritis [6], [7], [9], [10], [11], [8]. However, there has been no report on renal artery thrombosis associated with acute pyelonephritis and sepsis-induced DIC.
In sepsis-induced DIC, thrombus formation can be explained in three mechanisms and they are as follows: (1) upregulation of procoagulant pathway, (2) downregulation of physiologic anticoagulants, and (3) suppression of fibrinolysis. In this case, renal artery thrombosis occurred in the recovery phase of septic shock and thrombocytopenia. Increased platelet production can lead to increased immature platelet count and there is evidence that immature platelet fraction was increased in DIC [12]. The percentage and absolute number of reticulated platelet were elevated in patients with thrombosis [13]. As immature platelets have more COX-2 and thromboxane than normal platelets, the rapid platelet generation could have contributed to thrombogenesis. In addition, diabetes could have been an aggravating factor for platelet aggregation. Besides coagulopathy due to sepsis-associated DIC could have also contributed to the thrombus formation.
In cases of renal infarction, reperfusion therapy is useful within 12 h of onset time. Possible options for reperfusion therapy are intra-arterial thrombolytics, percutaneous angioplasty, systemic thrombolytics, and surgery. The success of intra-arterial thrombolytics and percutaneous angioplasty does not warrant recovery of renal function. Systemic thrombolytics may be helpful but have not been fully studied. Anticoagulation is still a controversial subject. In the process of getting informed consent from patient, we explained the treatment options, their possible side effects and complication. Considering the bleeding risk and possible unwanted results from thrombolytic therapy, we decided not to do thrombolytic therapy. A follow-up CT scan revealed improved renal perfusion and decreased Cr levels. If the thrombolytic therapy had been done in a timely manner, the improvement of kidney perfusion might have been more dramatic. The patient was discharged with improved general condition.
In this case, we encountered the matter of prophylactic anticoagulation during sepsis-induced DIC to prevent thrombosis. According to the Italian Society for Haemostasis and Thrombosis, the use of antithrombin, gabexate, plasma exchange, or thrombomodulin was not suggested for patients with DIC secondary to severe sepsis. Also, the use of unfractionated heparin or low-molecular weight heparin was not suggested except for thromboembolic prophylaxis in patients at high risk who do not have active bleeding [14]. Currently, activated protein C is the only approved therapy in the United States for sepsis complicated by DIC [15]. In this case, the patient was not at high risk for thrombosis. Therefore, prophylaxis was not administered.
Renal infarction is clinically important because it can compromise kidney function. However, diagnosis is not easy due to its nonspecific clinical presentation. In most cases of DIC, thrombosis occurs in venous system or small arteries. Therefore the possibility of renal infarct might be missed. This report suggests that in a patient with sepsis and improving thrombocytopenia, acute thrombosis can not only occur in microvessels, but can also occur in large arteries such as the renal artery. Therefore, we conclude that if there is a sudden onset of abdominal or flank pain, special concern regarding possible renal infarction is warranted.



 PDF Links
PDF Links PubReader
PubReader Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print
















