Introduction
Antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis (AAV) is characterized by necrotizing inflammation of small vessels and consists of a group of multisystemic diseases, such as microscopic polyangiitis, granulomatosis with polyangiitis, eosinophilic granulomatosis with polyangiitis, and renal limited vasculitis [
1,
2]. AAV commonly involves the kidneys and is a common cause of rapidly progressive glomerulonephritis [
3]. Despite treatment, renal survival and patient survival are poor in patients with ANCA-associated glomerulonephritis (AAGN) [
4,
5]. Prompt diagnosis and immediate proper immunosuppressive therapy are necessary to prevent the progression of end-stage kidney disease (ESKD). However, immunosuppression can also cause an increased in short-term or long-term mortality, mainly by infection [
6].
To prevent excessive immunosuppression and reduce complications, several studies have been conducted to find the histopathologic or clinical predictors for renal prognosis at the time of diagnosis. Various parameters, such as age, baseline renal function, percentage of normal/globally sclerotic glomeruli, and degree of interstitial fibrosis/tubular atrophy (IF/TA), have been identified as possible predictors [
4,
5,
7,
8]. However, they have limitations in predicting renal outcomes and have not been validated. Berden et al. [
9] developed a simple histopathologic classification of AAGN divided into four classes (focal, crescentic, mixed, and sclerotic) according to glomeruli and crescent types. More recently, Brix et al. [
10] accentuated the limitations of the histopathologic classification that reflects only glomerular findings. They developed a new classification according to the ANCA kidney risk scoring system that reflects not only histopathologic but also clinical findings.
Both classifications were developed using data from western AAV patients. Although there are Chinese and Japanese validation studies for histopathologic classification [
11,
12], no studies have used a Korean cohort. Also, there are still no studies on the ANCA kidney risk score for Asian AAGN patients. Therefore, this study aimed to evaluate the predictive value of the histopathologic and clinicopathologic classifications for renal outcomes among Korean AAGN patients.
Discussion
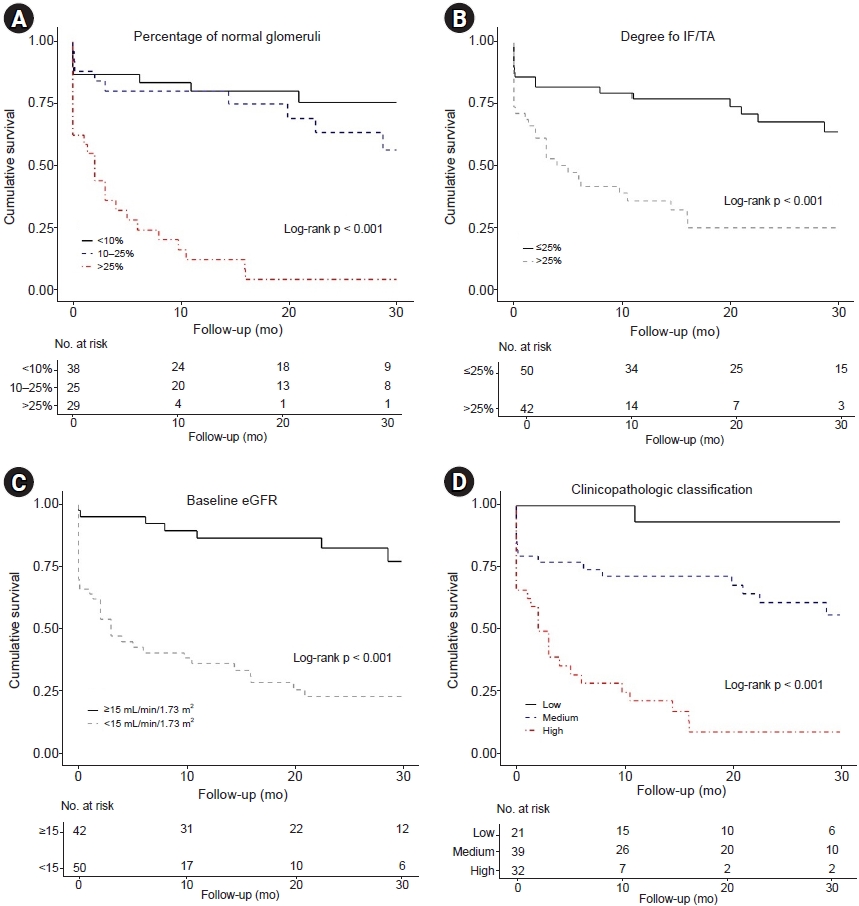
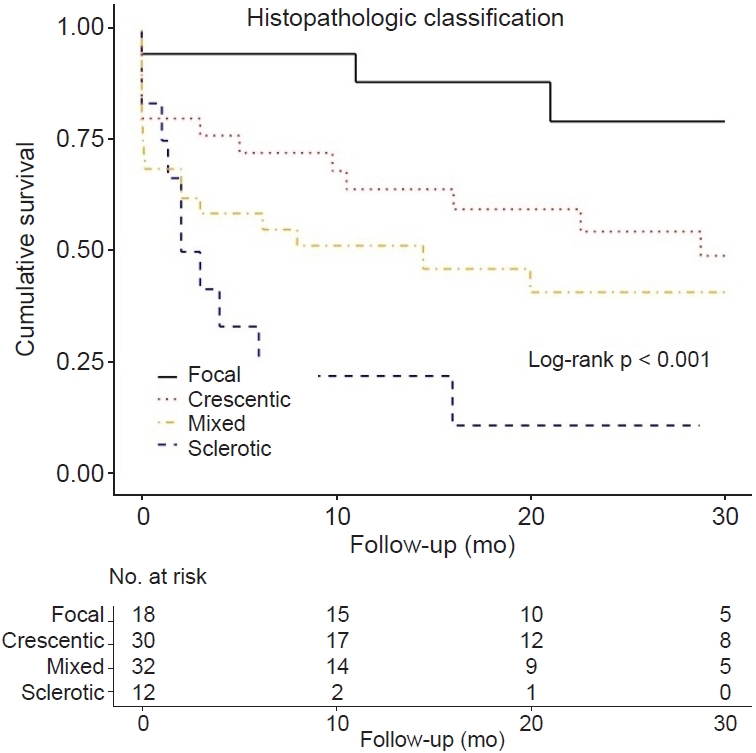
This study evaluated the renal prognostic value of both histopathologic and clinicopathologic classifications among Korean AAGN patients. Unlike in western countries [
9,
10,
18], the Korean AAGN cohort had a higher proportion of MPO-ANCA positive and higher incidence of progression to ESKD. Previous studies have shown that patients with MPO-ANCA have more abundant active and chronic lesions in renal histopathology than those of proteinase 3-ANCA positive patients. Therefore, MPO-ANCA positive patients have a higher risk of renal failure [
19–
21]. In the histopathologic classification according to glomerular pathology, the focal class had the best renal survival, and the mixed and sclerotic classes had the worse and worst renal survival, respectively. However, the crescentic class did not differ in renal survival compared to the focal class. In the clinicopathologic classification according to the percentage of normal glomeruli, degree of IF/TA, and baseline renal function, the high-risk group had poor renal survival than the low- or medium-risk group; however, there was no difference in renal survival between the low- and medium-risk groups. These results demonstrated that the classifications that originated from western countries were moderate in predicting renal outcomes in Asian AAGN patients. In particular, they showed high predictability for renal outcomes in the worst prognosis groups, such as the sclerotic class or the high-risk group.
AAGN is the most common manifestation of AAV and is highly associated with morbidity and mortality [
6,
20]. There are several known prognostic factors for progression to ESKD in AAGN, but it is difficult to predict renal outcomes accurately with any single factor [
5,
7,
10]. Berden et al. [
9] developed a simple histopathologic classification from European AAGN patients, which focused on the glomerular findings, except background tubulointerstitial findings and clinical findings. They reported that the focal class had a favorable outcome, the mixed class had an intermediate outcome, and the sclerotic class had the highest risk of not recovering renal function and progression to ESKD. Several western and Asian studies have also evaluated the prognostic value of the histopathologic classification [
6,
12,
20,
22,
23]. Most studies, including a recent meta-analysis, showed similar outcomes: the best outcome in the focal class and the worst outcome in the sclerotic class [
6,
12,
20,
22,
23]. Globally sclerotic glomeruli are not AAGN-specific findings. However, regardless of the AAGN disease activity, these chronic lesions indicate less response to treatment and a higher risk of progression to ESKD [
7,
24]. Unlike these classes, there is a controversy about the outcome of the crescentic and mixed classes. Some studies have reported that the crescentic class has a worse outcome than the focal or mixed class [
6,
23,
25]. Others have reported a non-inferior outcome of the crescentic class compared to the focal class because of a good therapeutic response [
9,
12,
20]. The results of this study are consistent with the latter—with Berden et al. [
9] and the Japanese cohort studies [
12]. Among Korean AAGN patients, the outcome of the crescentic class was relatively good and not inferior to the focal class. Recently, van Daalen et al. [
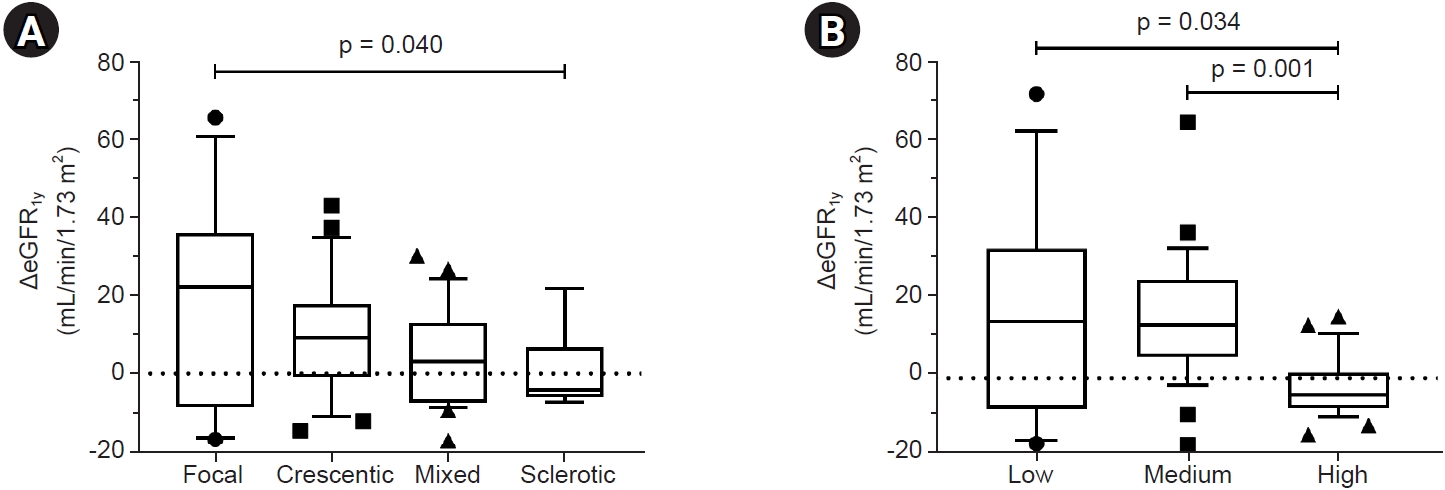
26] reported a validation study for AAGN from patients worldwide. The ΔeGFR
1y was significantly higher in the crescentic class than in the mixed class despite similar renal function at the time of diagnosis, indicating that the crescentic class had a better treatment response. They explained that this would be related to the different composition of crescent types—that the crescentic class is characterized by a majority of cellular crescents and these are reversible lesions, but the mixed class has more heterogeneous crescent types, such as fibrocellular and fibrous crescents, with poor response to treatment [
26,
27]. These results may also be related to the association of different human leukocyte antigen by ethnicity in AAV patients [
11]. Thus, based on the results of this study, Korean AAGN patients with the focal or crescentic class will need active treatment.
Brix et al. [
10] developed a more complex classification using a calculation based on the ANCA kidney risk score. All three factors included in this classification (normal glomeruli ratio, degree of IF/TA, and baseline renal function) are well-established prognostic markers of AAGN in previous studies [
4,
5,
7,
15,
24]. They determined the appropriate cutoff through rigorous analysis of the German AAGN cohort and divided the groups by scoring. The advantage of this classification is that it reflects both clinical laboratory (eGFR) and histopathologic (normal glomeruli and IF/TA) parameters. However, although these clinicopathologic variables were aggregated, the renal prognosis of the low- and medium-risk groups did not differ in the Korean cohort. A recent Turkish study also reported the same results as in this study [
28]. There are some possible reasons for this inconsistent result. First, the cutoff value of the percentage of normal glomeruli was too low. Berden et al. [
9] considered significance when the normal glomeruli percentage was higher than 50%, but Brix et al. [
10] set the cutoffs at a lower rate of 10% and 25%. Hilhorst et al. [
25] reported the worst survival in AAGN patients with normal glomeruli percentage of <25%. Tanna et al. [
5] reported a significant difference in renal survival among normal glomeruli percentages of 0% to 20%, 21% to 50%, and >51%. There was no difference in renal survival between normal glomeruli percentages of 10% to 25% and >25% in the results of this study. Therefore, a modified cutoff of the normal glomeruli ratio is required to accurately predict survival among Korean AAGN patients. Second, the proportion of patients who progressed to ESKD was much higher in this study. The cohort in this study had a lower baseline eGFR and a higher percentage of sclerotic glomeruli than the cohort studied by Brix et al. [
10], and the proportion of MPO-ANCA positive was also higher. All these indicators suggest a higher risk of progression to ESKD. Additionally, ethnicity may have possibly contributed to the difference in results from the previous study.
In addition to the aforementioned histopathologic classification, an analysis of the association between histopathologic findings reflecting active lesions and renal survival revealed that moderate to severe interstitial inflammation independently increased the risk of ESKD. This result is consistent with a previous study that reported diffuse interstitial infiltration of inflammatory cells is a predictor for poor renal outcome in European patients with AAGN [
4].
The incidence of AAGN increases with age. It is the second most common glomerulonephritis among Korean patients aged above 70 years who underwent renal biopsy [
29,
30]. AAGN patients have a high risk of not only renal failure but also death, so prompt diagnosis and proper management are necessary. However, the mortality of AAV patients within the first 12 months after diagnosis was as high as 11%, and half of them died from infection [
31]. Intensive immunosuppression also results in other adverse events, such as malignancy, bone marrow suppression, and gastrointestinal bleeding [
31]. Thus, especially in elderly patients who are vulnerable to the adverse effects of immunosuppressive therapy, it is important to accurately predict the therapeutic response and provide an appropriate intensity of treatment. Neither the histopathologic nor the clinicopathologic classification perfectly predicts renal outcomes in Korean AAGN patients. However, both classifications showed high predictability of ESKD progression for patients in either the sclerotic class or the high-risk group. In these patients, treatment intensity should be adjusted to consider the worse renal prognosis with less therapeutic response. Also, background histopathologic findings such as interstitial inflammation need to be considered more to accurately predict the renal outcome. In a recently published study on crescentic glomerulonephritis patients, severe arteriosclerosis and tertiary lymphoid organ formation are independent predictors for renal survival after adjusting for age and baseline renal function [
14]. By considering indicators that reflect chronic changes and inflammation activity, a more accurate prediction of renal outcomes will be possible.
The strength of this study is that it analyzed unselected patients who were diagnosed with AAGN at two university-based hospitals in Korea. For the first time in Korean AAGN patients, the predictive value of two European origin classifications was assessed. However, there are several limitations. First, this is a retrospective study, so there is a limitation to documenting the effectiveness of various treatments on renal outcomes. Second, the number of study patients was relatively small, although patients diagnosed with AAGN for a long period were included. This may reduce the validity. However, in both classifications, the number of patients was distributed appropriately in each class. Third, patients were treated at the discretion of the clinicians at each hospital. However, although there was no standard treatment, the Korean national health insurance system covers most medical expenses; therefore, treatment is performed according to the same national health insurance guidelines. Fourth, there is a possibility of sample bias. Partial tissue specimen cannot reflect the overall renal disease condition. However, like other AAGN studies [
5,
8], the bias was reduced by screening specimens that contained more than 10 glomeruli.
In conclusion, both histopathologic and clinicopathologic classifications were moderately able to predict the renal prognosis of Korean AAGN patients. For the sclerotic class or the high-risk group at diagnosis, the predictability for poor renal outcome was high due to poor response to treatment, and they had a high risk of progression to ESKD. Accordingly, clinicians need to balance the risks and benefits of treatment in Korean AAGN patients by identifying the group at high risk for renal outcomes.

















 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















