| Kidney Res Clin Pract > Volume 42(4); 2023 > Article |
|
Abstract
Background
Methods
Results
Conclusion
Notes
Funding
This study was supported by the Young Investigator Research Grant from the Korean Society of Nephrology (KSN 2019).
Figure 1.
Representative image of quantitative time-intensity curve parameters derived from contrast-enhanced ultrasonography.
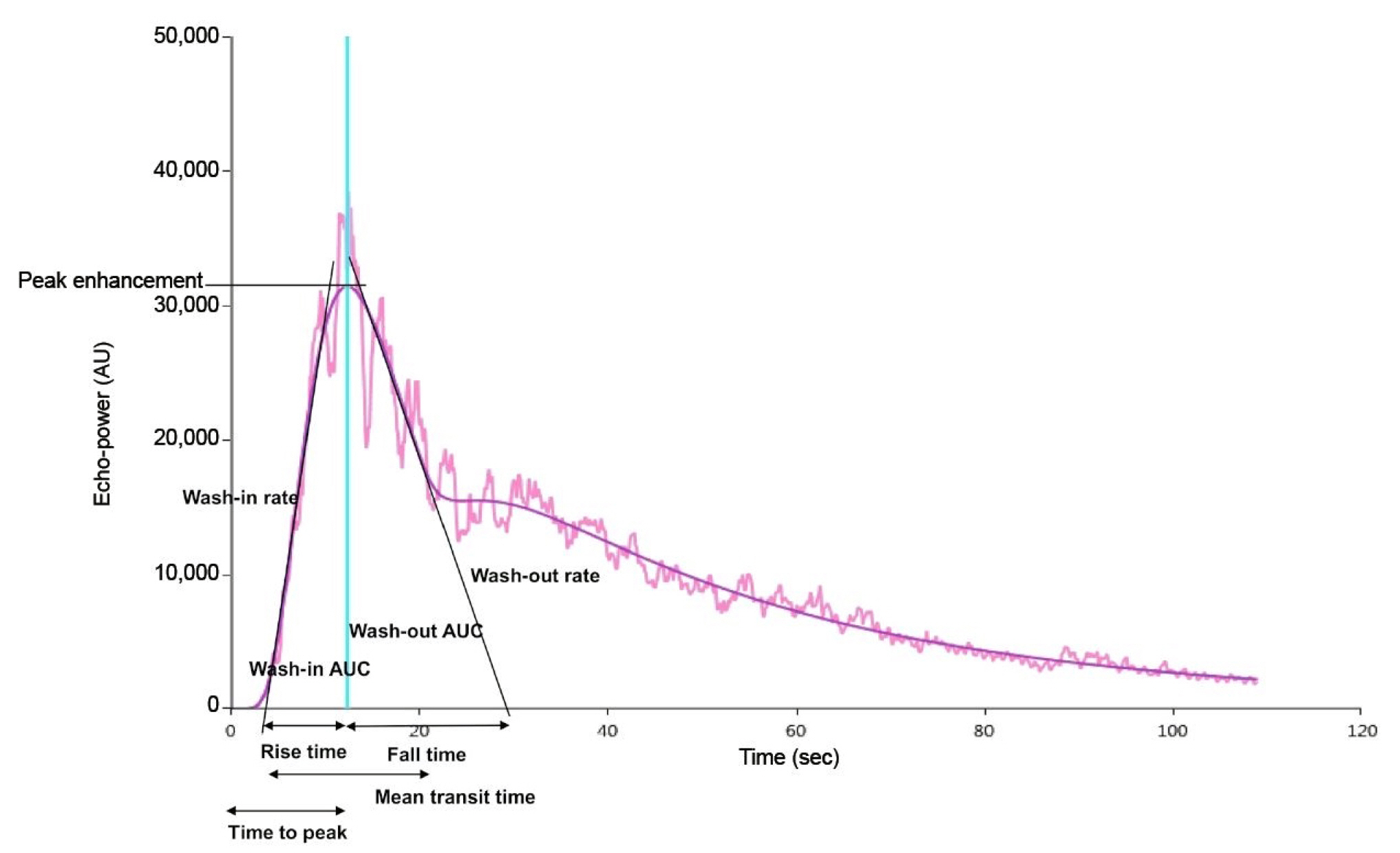
Figure 2.
Comparisons of contrast-enhanced ultrasonography-derived parameters between patients showing renal recovery and patients who did not recover.
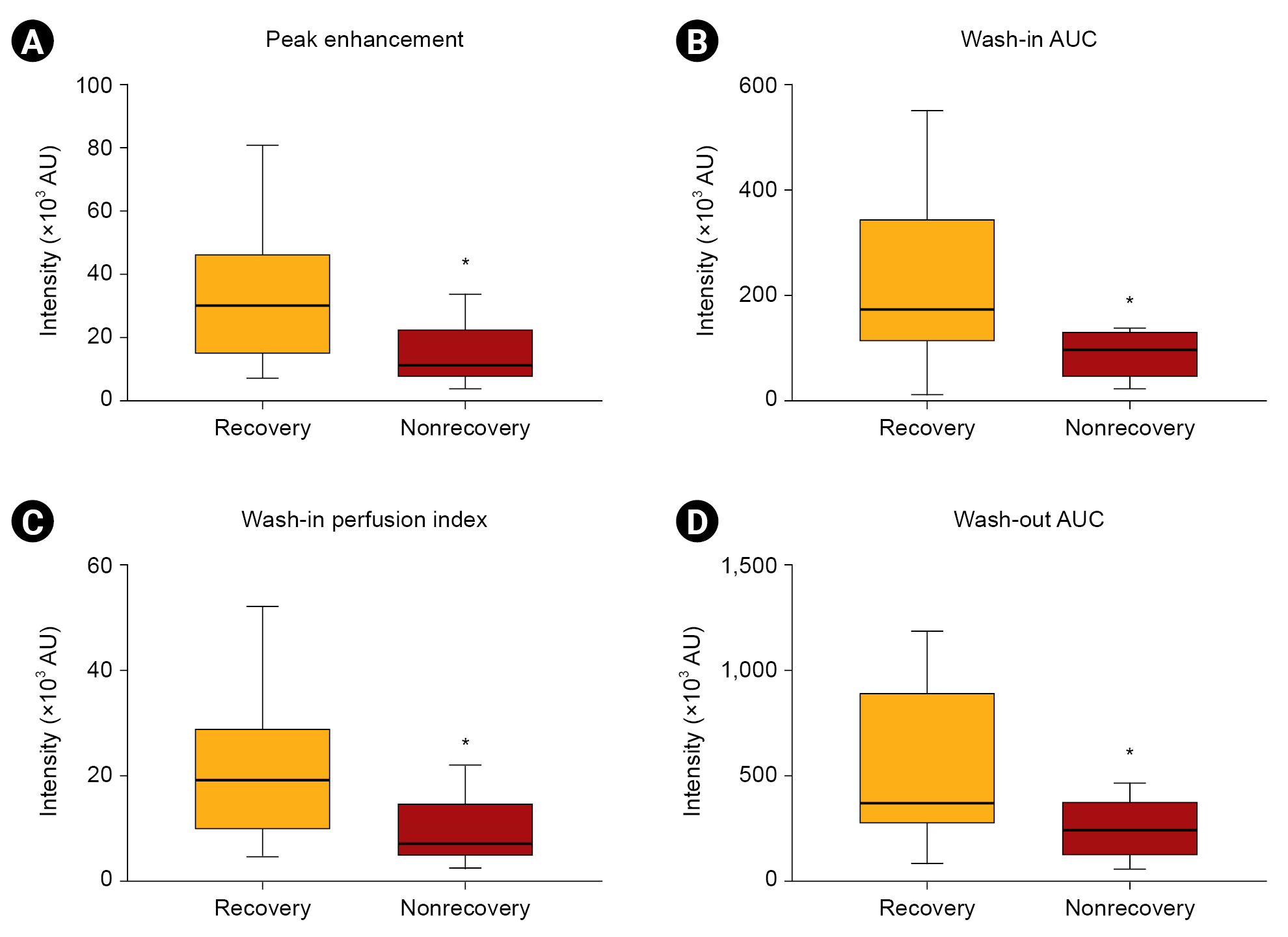
Figure 3.
The receiver operating characteristic curve for renal recovery using CEUS-derived parameters.
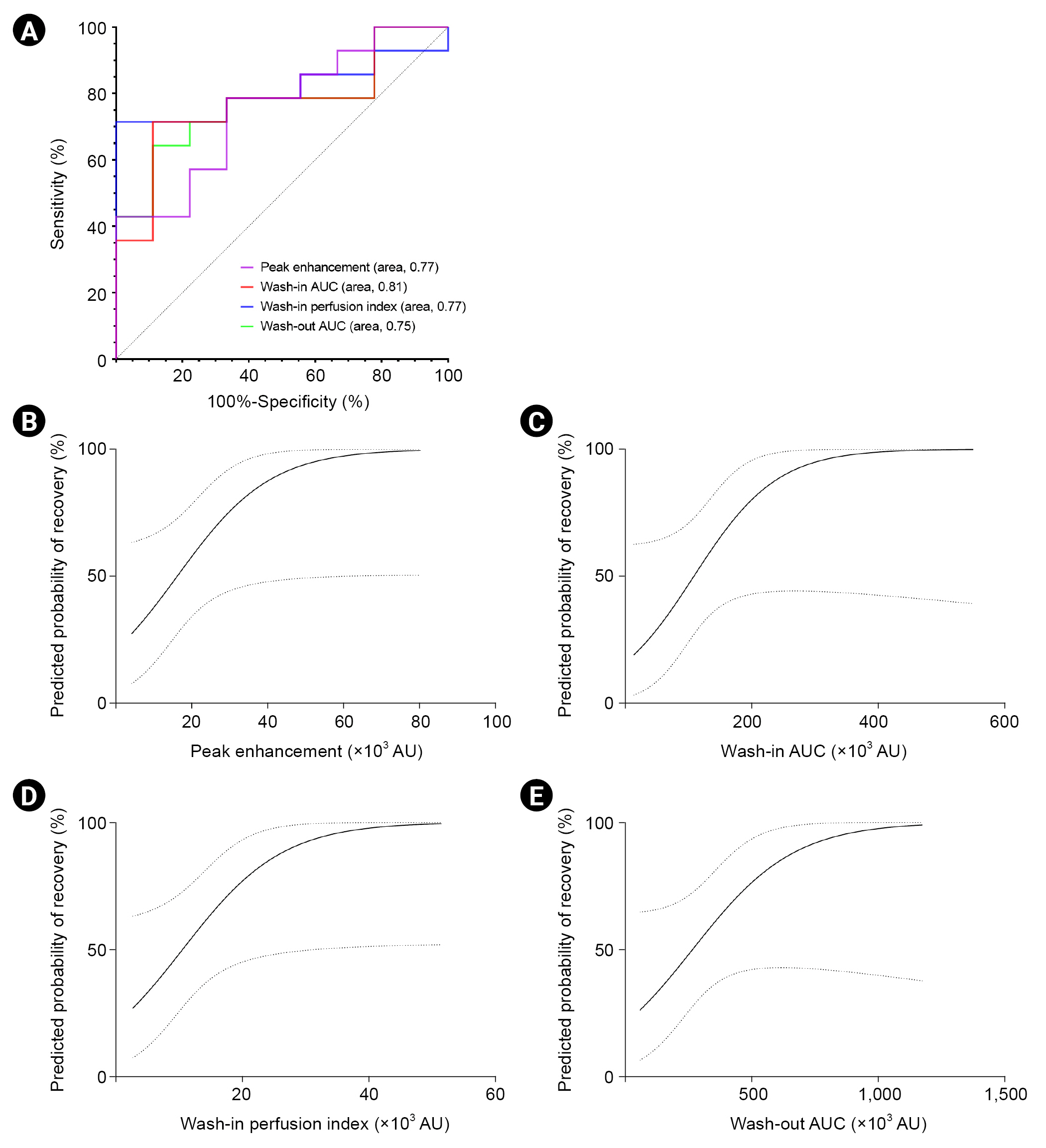
Table 1.
| Variable | Healthy group (n = 17) | CKD group (n = 18) | S-AKI group (n = 23) | p-value |
|---|---|---|---|---|
| Age (yr) | 53 (46–68) | 43 (35–52)* | 73 (65–87)*,† | <0.001 |
| Female sex | 5 (29.4) | 9 (50.0) | 9 (39.1) | 0.46 |
| Comorbidity | ||||
| Hypertension | 9 (52.9) | 15 (83.3) | 14 (60.9) | 0.14 |
| Diabetes mellitus | 2 (11.8) | 10 (55.6) | 14 (60.9) | 0.005 |
| Coronary heart disease | 0 (0) | 0 (0) | 3 (13.0) | 0.09 |
| Heart failure | 1 (5.9) | 0 (0) | 6 (26.1) | 0.03 |
| Liver disease | 0 (0) | 2 (11.1) | 1 (4.3) | 0.32 |
| Chronic lung disease | 1 (5.9) | 1 (5.6) | 2 (8.7) | 0.91 |
| Charlson comorbidity index | 0 (0–1) | 2 (0–2) | 2 (1–3) | <0.001 |
| Baseline renal function | ||||
| Creatinine (mg/dL) | 0.8 (0.6–0.9) | 1.7 (0.9–3.2) | 2.2 (1.1–2.9)a | 0.004 |
| eGFR (mL/min/1.73 m2) | 98.8 (92.7–108.8) | 45.2 (15.9–99.3) | 24.3 (14.4–62.3)a,* | 0.003 |
| Renal length (cm) | 10.7 (10.2–11.2) | 10.7 (10.3–11.4) | 10.3 (9.8–11.3) | 0.52 |
| Drug use | ||||
| RAS blocker | 4 (23.5) | 15 (83.3) | 7 (30.4) | <0.001 |
| NSAID | 1 (5.9) | 0 (0) | 0 (0) | 0.29 |
| CEUS parameter | ||||
| Peak enhancement (×103 AU) | 19.0 (13.6–42.0) | 13.0 (9.7–24.6) | 23.2 (8.5–33.3) | 0.54 |
| Wash-in AUC (×103 AU) | 117.0 (60.7–156.7) | 94.2 (65.3–183.6) | 136.8 (87.8–201.3) | 0.57 |
| Rise time (sec) | 7.6 (6.2–9.5) | 9.8 (7.6–12.5) | 10.0 (8.2–16.8)* | 0.03 |
| Mean transit time (sec) | 69.4 (48.5–107.5) | 59.6 (53.2–86.4) | 65.4 (48.9–92.2) | 0.84 |
| Time to peak (sec) | 11.7 (10.5–13.5) | 15.3 (12.7–17.3)* | 15.8 (12.5–21.1)* | 0.008 |
| Wash-in rate (×103 AU) | 3.3 (2.2–8.1) | 2.1 (1.5–4.2) | 3.2 (1.1–6.1) | 0.28 |
| Wash-in perfusion index (×103 AU) | 12.1 (8.5–27.3) | 8.7 (6.4–16.3) | 16.4 (5.4–21.4) | 0.55 |
| Wash-out AUC (×103 AU) | 246.3 (147.2–307.6) | 249.4 (169.9–532.9) | 314.3 (229.1–571.7) | 0.25 |
| Fall time (sec) | 17.4 (10.9–21.6) | 22.1 (15.3–33.5) | 22.3 (17.2–40.3) | 0.09 |
| Wash-out rate (×103 AU) | 1.1 (0.9–3.3) | 0.7 (0.3–1.3) | 0.7 (0.3–2.8) | 0.19 |
Data are expressed as median (interquartile range), number (%), or mean ± standard deviation.
AU, arbitrary unit; AUC, area under the curve; CEUS, contrast-enhanced ultrasonography; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; NSAID, non-steroidal anti-inflammatory drug; RAS, renin-angiotensin system; RRT, renal replacement therapy; S-AKI, sepsis-associated acute kidney injury.
Table 2.
| Variable |
Parameter relating to microvascular blood volume and flow |
Parameter relating to microvascular flow velocity |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Peak enhancement | Wash-in AUC | Wash-in rate | Wash-in perfusion index | Wash-out AUC | Wash-out rate | Rise time | Mean transit time | Time to peak | Fall time | |
| Age | 0.03 | 0.17 | −0.01 | 0.03 | 0.14 | −0.09 | 0.22 | 0.05 | 0.19 | 0.17 |
| Sex | 0.13 | 0.17 | 0.16 | 0.14 | 0.25 | 0.04 | 0.13 | −0.14 | 0.02 | 0.28 |
| Hypertension | −0.21 | −0.23 | −0.13 | −0.20 | −0.21 | −0.13 | 0.12 | −0.03 | 0.10 | 0.05 |
| Diabetes mellitus | −0.13 | −0.09 | −0.11 | −0.13 | −0.04 | −0.12 | −0.10 | 0.04 | −0.17 | −0.05 |
| Heart failure | 0.19 | 0.16 | 0.16 | 0.19 | 0.07 | 0.13 | 0.13 | 0.06 | 0.21 | −0.03 |
| Charlson comorbidity index | 0.02 | −0.02 | 0.02 | 0.02 | −0.11 | 0.02 | −0.10 | 0.03 | −0.05 | −0.19 |
| Renal length | 0.09 | 0.01 | 0.14 | 0.09 | 0.09 | 0.14 | −0.15 | −0.10 | −0.22 | −0.13 |
| At the time of RRT commencement | ||||||||||
| SOFA | 0.13 | 0.05 | 0.21 | 0.14 | 0.19 | 0.19 | −0.24 | −0.13 | −0.36 | −0.01 |
| Lactate level | −0.10 | 0.06 | −0.12 | −0.08 | 0.17 | −0.12 | 0.36 | 0.19 | 0.28 | 0.49* |
| Septic shock | 0.17 | 0.18 | 0.15 | 0.17 | 0.29 | 0.13 | −0.13 | −0.03 | −0.21 | 0.07 |
| Urine output | 0.18 | 0.20 | 0.10 | 0.18 | 0.06 | 0.13 | −0.21 | −0.15 | −0.20 | −0.20 |
| At the time of CEUS | ||||||||||
| Mean blood pressure | −0.24 | −0.20 | −0.20 | −0.23 | −0.23 | −0.19 | −0.01 | −0.28 | −0.08 | 0.05 |
| RAS blocker | −0.05 | 0.14 | −0.16 | −0.04 | 0.19 | −0.19 | 0.30 | −0.27 | 0.19 | 0.37 |
| Urine output | 0.36 | 0.41 | 0.24 | 0.37 | 0.35 | 0.21 | −0.13 | −0.15 | −0.18 | −0.05 |
| Laboratory results | ||||||||||
| Hemoglobin | −0.25 | −0.20 | −0.19 | −0.25 | −0.18 | −0.25 | 0.04 | −0.07 | −0.03 | 0.11 |
| Albumin | −0.20 | −0.10 | −0.25 | −0.20 | −0.12 | −0.23 | 0.31 | 0.17 | 0.35 | 0.14 |
| C-reactive protein | 0.03 | 0.23 | −0.09 | 0.05 | 0.34 | −0.15 | 0.17 | 0.41* | 0.11 | 0.15 |
| Total bilirubin | 0.05 | 0.02 | 0.16 | 0.05 | −0.09 | 0.16 | 0.03 | 0.20 | 0.09 | −0.09 |
Data are expressed as correlation coefficient derived from the Pearson correlation analysis.
AUC, area under the curve; CEUS, contrast-enhanced ultrasonography; RAS, renin-angiotensin system; RRT, renal replacement therapy; S-AKI, sepsis-associated acute kidney injury; SOFA, Sequential Organ Failure Assessment.
Table 3.
Data are expressed as median (interquartile range) or number (%).
CEUS, contrast-enhanced ultrasonography; eGFR, estimated glomerular filtration rate; RAS, renin-angiotensin system; RRT, renal replacement therapy; S-AKI, sepsis-associated acute kidney injury; SOFA, Sequential Organ Failure Assessment.
Table 4.
| Variable |
Univariate |
Multivariatea | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 0.97 (0.90–1.04) | 0.32 | 0.94 (0.85–1.05) | 0.27 |
| Female sex | 1.50 (0.26–8.58) | 0.65 | 3.61 (0.29–44.94) | 0.32 |
| Charlson comorbidity index | 0.84 (0.54–1.30) | 0.43 | 0.93 (0.57–1.51) | 0.76 |
| SOFA score | 1.19 (0.90–1.58) | 0.23 | 1.08 (0.78–1.50) | 0.64 |
| CEUS parameters (per 1 × 103 AU) | ||||
| Peak enhancement | 1.09 (1.00–1.18) | 0.05 | 1.08 (0.99–1.17) | 0.09 |
| Wash-in AUC | 1.02 (1.00–1.03) | 0.07 | 1.02 (1.00–1.04) | 0.07 |
| Wash-in perfusion index | 1.14 (1.00–1.29) | 0.05 | 1.12 (0.98–1.27) | 0.09 |
| Wash-out AUC | 1.01 (1.00–1.01) | 0.08 | 1.01(1.00–1.01) | 0.08 |
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 2,047 View
- 99 Download
- ORCID iDs
-
Jungho Shin

https://orcid.org/0000-0001-9755-3100Jin Ho Hwang

https://orcid.org/0000-0003-0829-0922Sung Bin Park

https://orcid.org/0000-0002-4155-9260Su Hyun Kim

https://orcid.org/0000-0001-8608-9525 - Related articles
-
Renal Recovery from Severe Acute Kidney Injury Requiring Renal Replacement Therapy2009 July;28(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















