| Kidney Res Clin Pract > Epub ahead of print |
Abstract
Background
Methods
Results
Supplementary Materials
Notes
Funding
This work was supported by the Guangdong Provincial Key Laboratory of Nephrology (grant No. 2020B1212060028) and the NHC Key Laboratory of Clinical Nephrology (Sun Yat-Sen University).
Data sharing statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Authors’ contributions
Conceptualization, Funding acquisition: XY
Data curation, Resources, Supervision: XY, HM, FH, QG, HY
Formal analysis: RL, JG, HY
Investigation: RL, YP, HW
Writing–original draft: RL, JG
Writing–review & editing: RL, JG, XY
All authors approved the final version of the manuscript.
Acknowledgments
Figure 1.
Flow chart of the study population.
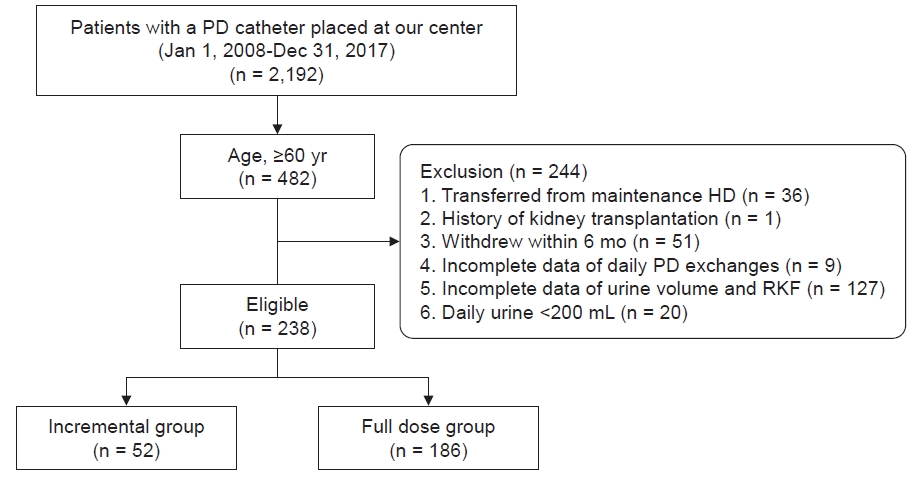
Figure 2.
Kaplan-Meier curve of anuria-free survival for incremental PD over full dose PD in older patients.

Figure 3.
Kaplan-Meier curves of survival rate between incremental group and full dose group in older patients.

Figure 4.
Kaplan-Meier curve of technique failure-free survival between incremental group and full dose group in older patients.
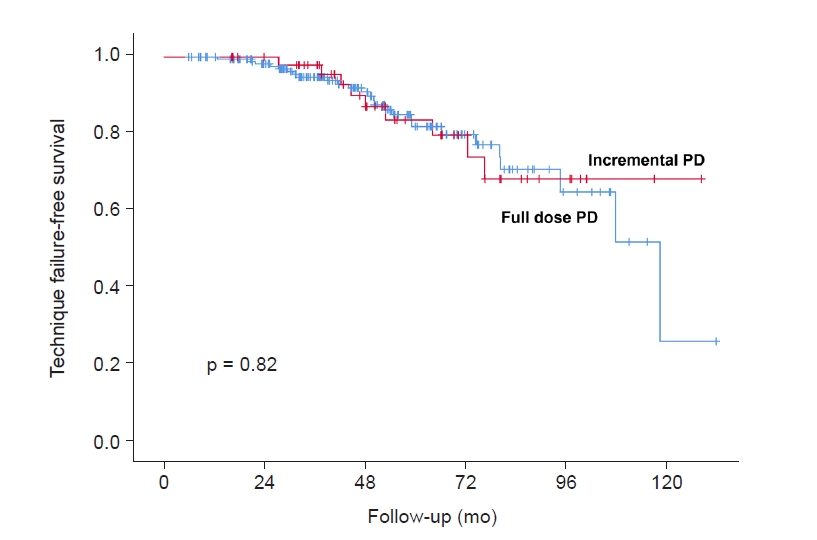
Table 1.
| Characteristic | All | Full dose group | Incremental group | p-value |
|---|---|---|---|---|
| No. of patients | 238 | 186 | 52 | |
| Age (yr) | 67.8 ± 5.7 | 68.0 ± 5.6 | 67.2 ± 6.0 | 0.37 |
| Female sex | 109 (45.8) | 84 (45.2) | 25 (48.1) | 0.75 |
| Body mass index (kg/m2) | 22.0 ± 3.0 | 22.2 ± 2.9 | 21.6 ± 2.9 | 0.22 |
| Primary kidney disease | 0.995 | |||
| Glomerulonephritis | 63 (26.5) | 49 (26.3) | 14 (26.9) | |
| Diabetic nephropathy | 109 (45.8) | 86 (46.2) | 23 (44.2) | |
| Renal vascular disease | 38 (16.0) | 29 (15.6) | 9 (17.3) | |
| Others | 28 (11.8) | 22 (11.8) | 6 (11.5) | |
| Diabetes | 131 (55.0) | 105 (56.5) | 26 (50.0) | 0.43 |
| Cardiovascular disease | 170 (71.0) | 135 (72.6) | 34 (65.4) | 0.31 |
| Hemoglobin (g/L) | 106 ± 18 | 106 ± 17 | 108 ± 21 | 0.52 |
| Albumin (g/L) | 35.0 ± 4.1 | 34.9 ± 4.1 | 35.4 ± 4.2 | 0.46 |
| SBP (mmHg) | 140 ± 16 | 141 ± 16 | 138 ± 15 | 0.23 |
| DBP (mmHg) | 77 ± 10 | 76 ± 10 | 78 ± 11 | 0.33 |
| Calcium (mmol/L) | 2.25 ± 0.21 | 2.26 ± 0.22 | 2.20 ± 0.18 | 0.05 |
| Phosphorus (mmol/L) | 1.25 ± 0.32 | 1.23 ± 0.31 | 1.29 ± 0.33 | 0.24 |
| TC (mmol/L) | 5.18 ± 1.28 | 5.22 ± 1.32 | 5.02 ± 1.09 | 0.32 |
| Triglyceride (mmol/L) | 1.47 (1.02–2.14) | 1.53 (1.11–2.23) | 1.21 (0.8 –1.82) | 0.009* |
| sCr (μmol/L) | 699 ± 233 | 717 ± 241 | 636 ± 194 | 0.03* |
| BUN (mmol/L) | 14.7 ± 5.0 | 14.3 ± 4.7 | 16.1 ± 5.8 | 0.02* |
| Uric acid (μmol/L) | 394 ± 88 | 393 ± 85 | 398 ± 97 | 0.69 |
| iPTH (pg/mL) | 189(83–353) | 176 (67–349) | 240 (141–421) | 0.02* |
| nPCR (g/kg/day) | 0.89 ± 0.24 | 0.86 ± 0.23 | 1.01 ± 0.26 | <0.001* |
| Glucose exposure (g/day) | 133.0 ± 23.2 | 138.4 ± 21.3 | 113.6 ± 19.2 | <0.001* |
| CrCl (L/wk/1.73 m2) | ||||
| Total | 90.9 ± 25.2 | 90.4 ± 25.9 | 92.9 ± 22.8 | 0.52 |
| Renal | 44.8 ± 25.3 | 41.8 ± 24.6 | 55.7 ± 25.1 | <0.001* |
| Peritoneal | 46.1 ±10.7 | 48.5 ± 9.7 | 37.2 ± 9.5 | <0.001* |
| Kt/V | ||||
| Total | 2.63 ± 0.68 | 2.65 ± 0.69 | 2.58 ± 0.65 | 0.51 |
| Renal | 0.92 ± 0.54 | 0.84 ± 0.51 | 1.20 ± 0.55 | <0.001* |
| Peritoneal | 1.72 ± 0.47 | 1.81 ± 0.45 | 1.40 ± 0.37 | <0.001* |
| RKF (mL/min/1.73 m2) | 4.15 ± 2.39 | 3.90 ± 2.32 | 5.06 ± 2.41 | 0.002* |
| Urine (mL/day) | 700 (400–1,000) | 600 (385–1,000) | 820 (600–1,200) | 0.002* |
| 24 hr-ultrafiltration (mL) | 350 (100–600) | 400 (175–600) | 200 (–100 to 425) | 0.001* |
| Daily dwell volume (L/day) | 7.6 ± 1.0 | 8.1 ± 0.5 | 5.9 ± 0.4 | <0.001* |
Data are expressed as number only, mean ± standard deviation, number (%), or median (interquartile range).
BUN, blood urea nitrogen; CrCl, weekly creatinine clearance; DBP, diastolic blood pressure; iPTH, intact parathyroid hormone; Kt/V, urea clearance normalized to total body water; nPCR, normalized protein catabolic rate; RKF, residual kidney function; SBP, systolic blood pressure; sCr, serum creatinine; TC, total cholesterol.
Table 2.
| Group | No. of patients | No. of events | HR (95% CI) | p-value |
|---|---|---|---|---|
| Anuria | ||||
| Model 1 | 238 | 75 | 0.47 (0.27–0.84) | 0.01 |
| Model 2a | 238 | 75 | 0.49 (0.26–0.90) | 0.02 |
| Model 3b | 238 | 75 | 0.44 (0.24–0.81) | 0.008 |
| All-cause mortality | ||||
| Model 1 | 238 | 124 | 0.53 (0.33–0.86) | 0.009 |
| Model 2c | 238 | 124 | 0.55 (0.34–0.92) | 0.02 |
| Model 3d | 238 | 124 | 0.59 (0.36–0.98) | 0.04 |
| CV mortality | ||||
| Model 1 | 238 | 72 | 0.41 (0.20–0.82) | 0.01 |
| Model 2e | 238 | 72 | 0.42 (0.20–0.87) | 0.02 |
| Model 3f | 238 | 72 | 0.51 (0.25–1.05) | 0.07 |
a Adjusted for sex, body mass index (BMI), hemoglobin (Hb), albumin (Alb), diastolic blood pressure (DBP), normalized protein catabolic rate (nPCR), and residual kidney function (RKF).
c Adjusted for sex, BMI, present of diabetes, history of cardiovascular disease (CVD), DBP, blood urea nitrogen (BUN), Hb, Alb, nPCR, and RKF.
Table 3.
| Follow-up (mo) | Peritonitis rate (episodes per person-year) | p-value | ||
|---|---|---|---|---|
| All |
Full dose group |
Incremental group |
||
| 12 | 0.191 | 0.217 | 0.096 | 0.07 |
| 24 | 0.180 | 0.192 | 0.137 | 0.25 |
| 36 | 0.178 | 0.197 | 0.115 | 0.03* |
| 48 | 0.186 | 0.201 | 0.136 | 0.07 |
| 60 | 0.198 | 0.211 | 0.158 | 0.13 |
| 132 | 0.196 | 0.203 | 0.175 | 0.39 |
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 1,315 View
- 51 Download
- ORCID iDs
-
Ruihua Liu

https://orcid.org/0000-0003-3998-3545Jing Guo

https://orcid.org/0000-0003-2039-6199Hongjian Ye

https://orcid.org/0000-0002-9565-508XYuan Peng

https://orcid.org/0000-0001-7625-438XHaishan Wu

https://orcid.org/0000-0002-3070-3925Haiping Mao

https://orcid.org/0000-0002-3608-3851Qunying Guo

https://orcid.org/0000-0002-0252-6570Fengxian Huang

https://orcid.org/0000-0003-0022-3784Xiao Yang

https://orcid.org/0000-0003-0437-2015 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















