| Kidney Res Clin Pract > Epub ahead of print |
Abstract
Background
Methods
Results
Notes
Data sharing statement
The data presented in this study are available on request from the corresponding author.
AuthorsŌĆÖ contributions
Conceptualization, Formal analysis, Methodology, Software: GY, HJC
Data curation, Supervision, Project administration: HJC
Investigation, Visualization: GY
Validation: EJK, SP, JCJ, SK, KYN, HJC
Writing ŌĆō original draft: GY
Writing ŌĆō review & editing: GY, HJC
All authors read and approved the final manuscript.
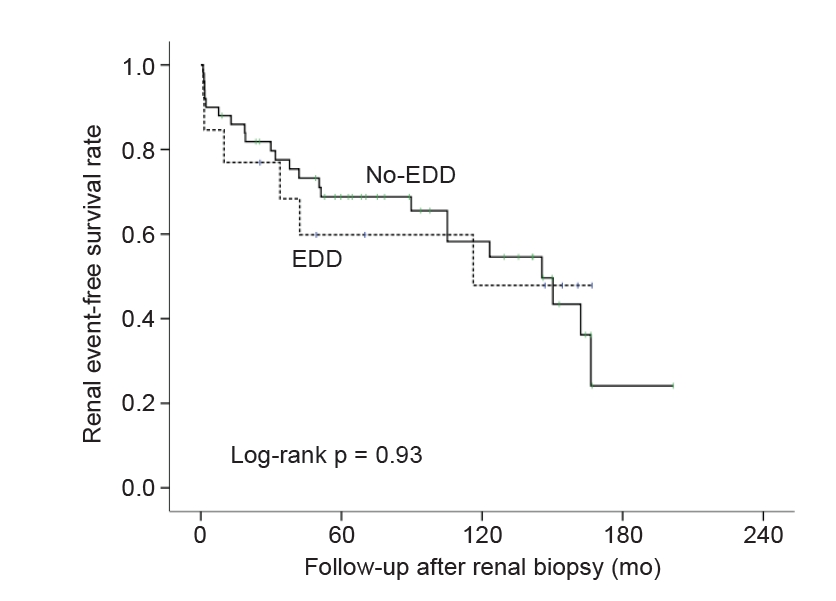
Figure┬Ā1.
Renal event-free survival rate of patients with immunoglobulin M nephropathy according to the presence of electron-dense deposits on mesangium.
Figure┬Ā2.
Renal event-free survival rate of patients with MCD, FSGS, IgAN, and IgMN.

Table┬Ā1.
Data are expressed as mean ┬▒ standard deviation, number (%), or median (interquartile range). The p-value by Mann-Whitney test for continuous variables and chi-square test for categorical variables.
AKI, acute kidney injury based on the lowest creatinine value during the follow-up period; CHD, coronary heart disease; DBP, diastolic blood pressure; EDD, electron-dense deposits; eGFR, estimated glomerular filtration rate by CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation; HPF, high-power field; IF, immunofluorescent; Ig, immunoglobulin; NO-EDD, without EDD; RBC, red blood cell; SBP, systolic blood pressure; UPCR, spot urine protein-to-creatinine ratio; uc, unable to calculate.
Table┬Ā2.
| Pathologic diagnosis | Completeness of data (%) | EDD group (n = 13) | NO-EDD group (n = 50) | p-value |
|---|---|---|---|---|
| FU after biopsya (mo) | 100 | 57.4 ┬▒ 61.3 | 81.6 ┬▒ 64.3 | 0.18 |
| No. of laboratory tests during FU (/mo) | 100 | 1.6 ┬▒ 2.3 | 1.7 ┬▒ 3.1 | 0.67 |
| UPCR at last visit (g/g Cr) | 96.8 | 0.91 (0.15ŌĆō2.11) | 0.91 (0.14ŌĆō2.57) | |
| ŌĆā<0.30 | 4 (30.8) | 15 (31.3) | ||
| ŌĆā0.30ŌĆō2.99 | 7 (53.8) | 22 (45.8) | 0.81 | |
| ŌĆāŌēź3.00 | 2 (15.4) | 11 (22.9) | ||
| Creatinine at last visit (mg/dL) | 100 | 1.35 (0.91ŌĆō2.97) | 1.25 (0.85ŌĆō3.15) | 0.83 |
| eGFR at last visit (mL/min/1.73 m2) | 100 | 48.3 (17.9ŌĆō94.6) | 62.9 (18.7ŌĆō84.8) | 0.95 |
| Treatment after biopsy | ||||
| ŌĆāRAS blocker | 100 | 11 (84.6) | 44 (88.0) | 0.67 |
| ŌĆāAntihypertensive medication | 100 | 12 (92.3) | 45 (90.0) | >0.99 |
| ŌĆāAntidiabetic medication | 100 | 7 (53.8) | 17 (34.0) | 0.21 |
| Immunosuppressive treatment after biopsy | 100 | 7 (53.8) | 29 (58.0) | >0.99 |
| ŌĆāSteroid only | 100 | 4 (30.8) | 13 (26.0) | |
| ŌĆāSteroid and cyclophosphamide | 100 | 3 (23.1) | 5 (10.0) | 0.22 |
| ŌĆāSteroid and other immunosuppressantsb | 100 | 0 (0) | 11 (22.0) | |
| Outcomes | ||||
| ŌĆāRenal eventc during FU | 100 | 6 (46.2) | 23 (46.0) | >0.99 |
| ŌĆāESRD during FU | 95.2 | 2 (15.4) | 10 (21.3) | >0.99 |
| ŌĆāDeath during FU | 100 | 1 (7.7) | 4 (8.0) | >0.99 |
Data are expressed as mean ┬▒ standard deviation, median (interquartile range), or number (%). p-value by Mann-Whitney test for continuous variables and chi-square test for categorical variables.
EDD, electron-dense deposits; ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; FU, follow-up; NO-EDD, without EDD; RAS, renin-angiotensin-aldosterone; UPCR, urine protein-to-creatinine ratio.
Table┬Ā3.
| Characteristic | IgMN group | FSGS group | MCD group | IgAN group | p-value* | p-value** | p-value*** |
|---|---|---|---|---|---|---|---|
| Pathologic diagnosis | |||||||
| ŌĆāNo. of patients | 63 | 103 | 91 | 469 | |||
| ŌĆāAge (yr) | 55.3 ┬▒ 18.8 | 52.2 ┬▒ 17.7 | 51.7 ┬▒ 19.8 | 43.4 ┬▒ 14.6 | 0.29 | 0.25 | <0.001 |
| ŌĆāMale sex | 36 (57.1) | 52 (50.5) | 48 (52.7) | 215 (45.8) | 0.43 | 0.62 | 0.11 |
| ŌĆāDiabetes melliuts | 17 (27.4) | 16 (15.8) | 14 (15.7) | 19 (4.1) | 0.11 | 0.1 | <0.001 |
| ŌĆāHypertension | 43 (68.3) | 78 (75.7) | 46 (50.5) | 266 (56.7) | 0.37 | 0.03 | 0.10 |
| ŌĆāHistory of CHD | 11 (17.5) | 12 (11.7) | 4 (4.4) | 17 (3.6) | 0.36 | 0.01 | <0.001 |
| ŌĆāSBP (mmHg) | 134 ┬▒ 21 | 132 ┬▒ 19 | 123 ┬▒ 16 | 126 ┬▒ 17 | 0.70 | 0.00 | 0.008 |
| ŌĆāDBP (mmHg) | 75 ┬▒ 12 | 76 ┬▒ 11 | 72 ┬▒ 12 | 73 ┬▒ 12 | 0.39 | 0.22 | 0.36 |
| ŌĆāCholesterol (mg/dL) | 228 ┬▒ 111 | 237 ┬▒ 104 | 320 ┬▒ 135 | 190 ┬▒ 46 | 0.62 | <0.001 | 0.009 |
| ŌĆāGlucose (mg/dL) | 121 ┬▒ 44 | 122 ┬▒ 46 | 112 ┬▒ 35 | 111 ┬▒ 27 | 0.87 | 0.18 | 0.08 |
| ŌĆāProtein (g/dL) | 5.9 ┬▒ 1.2 | 6.2 ┬▒ 1.2 | 4.9 ┬▒ 1.2 | 6.6 ┬▒ 0.7 | 0.15 | <0.001 | <0.001 |
| ŌĆāAlbumin (g/dL) | 3.3 ┬▒ 0.8 | 3.4 ┬▒ 0.8 | 2.6 ┬▒ 1.0 | 3.2 ┬▒ 0.5 | 0.19 | <0.001 | <0.001 |
| ŌĆāHemoglobin (g/dL) | 12.8 ┬▒ 2.8 | 12.9 ┬▒ 2.3 | 13.6 ┬▒ 1.9 | 12.9 ┬▒ 1.8 | 0.72 | 0.045 | 0.66 |
| ŌĆāCreatinine (mg/dL) | 1.87 ┬▒ 2.32 | 1.41 ┬▒ 1.22 | 1.97 ┬▒ 1.04 | 1.12 ┬▒ 0.75 | 0.14 | 0.03 | 0.01 |
| ŌĆāeGFR (mL/min/1.73 m2) | 67 ┬▒ 39 | 70 ┬▒ 34 | 83 ┬▒ 36 | 84 ┬▒ 34 | 0.63 | 0.01 | <0.001 |
| ŌĆāAKI at biopsy | 26 (41.3) | 35 (34.0) | 37 (40.7) | 108 (23.0) | 0.41 | >0.99 | 0.003 |
| ŌĆāŌĆāStage 1 | 11 (17.5) | 23 (22.3) | 14 (15.4) | 80 (17.1) | |||
| ŌĆāŌĆāStage 2 | 6 (9.5) | 7 (6.8) | 9 (9.9) | 22 (4.7) | 0.15 | 0.99 | <0.001 |
| ŌĆāŌĆāStage 3 | 9 (14.3) | 5 (4.9) | 14 (15.4) | 6 (1.3) | |||
| ŌĆāHematuria (RBC, Ōēź5/HFP) | 34 (54.8) | 54 (55.7) | 34 (39.5) | 370 (82.4) | >0.99 | 0.07 | <0.001 |
| ŌĆāUPCR at biopsy (g/g Cr) | 3.63 ┬▒ 5.43 | 3.70 ┬▒ 4.23 | 6.22 ┬▒ 6.39 | 1.61 ┬▒ 1.96 | 0.93 | 0.01 | <0.001 |
| ŌĆāŌĆā<0.30 | 2 (3.3) | 0 (0) | 0 (0) | 1 (0.2) | |||
| ŌĆāŌĆā0.30ŌĆō2.99 | 40 (65.6) | 57 (55.9) | 35 (39.3) | 401 (86.6) | 0.06 | 0.001 | <0.001 |
| ŌĆāŌĆāŌēź3.00 | 19 (31.1) | 45 (44.1) | 54 (60.7) | 61 (13.2) | |||
| Renal pathology | |||||||
| ŌĆāLight microscopic findings | |||||||
| ŌĆāŌĆāGlomeruli | |||||||
| ŌĆāŌĆāŌĆāNo. of glomeruli | 32.3 ┬▒ 22.9 | 30.2 ┬▒ 16.9 | 35.0 ┬▒ 18.5 | 34.5 ┬▒ 20.5 | 0.496 | 0.42 | 0.44 |
| ŌĆāŌĆāŌĆāGlobal sclerosis (%) | 24.0 ┬▒ 22.9 | 27.1 ┬▒ 23.1 | 8.6 ┬▒ 13.2 | 22.8 ┬▒ 20.7 | 0.41 | <0.001 | 0.66 |
| ŌĆāŌĆāŌĆāSegmental sclerosis (%) | 6.4 ┬▒ 10.6 | 8.7 ┬▒ 7.4 | 0.0 ┬▒ 0.0 | 6.8 ┬▒ 8.4 | 0.13 | <0.001 | 0.76 |
| ŌĆāŌĆāŌĆāCrescent (%) | 1.9 ┬▒ 9.9 | 0.0 ┬▒ 0.0 | 0.0 ┬▒ 0.0 | 1.8 ┬▒ 4.8 | 0.14 | 0.14 | 0.89 |
| ŌĆāŌĆāŌĆāIschemic change (%) | 0.0 ┬▒ 0.0 | 0.0 ┬▒ 0.2 | 0.2 ┬▒ 1.0 | 0.2 ┬▒ 2.9 | 0.44 | 0.15 | 0.50 |
| ŌĆāŌĆāŌĆāMesangial hypercellularity | 21 (33.3) | 23 (22.3) | 7 (7.7) | 454 (97.2) | 0.15 | <0.001 | <0.001 |
| ŌĆāŌĆāŌĆāIncrease of mesangial matrix | 17 (27.0) | 15 (14.6) | 11 (12.1) | 429 (91.9) | 0.07 | 0.03 | <0.001 |
| ŌĆāŌĆāTubulointerstitium | |||||||
| ŌĆāŌĆāŌĆāTubular atrophy | 54 (85.7) | 97 (94.2) | 68 (74.7) | 448 (95.9) | 0.09 | 0.11 | <0.001 |
| ŌĆāŌĆāŌĆāInterstitial fibrosis | 52 (82.5) | 95 (92.2) | 67 (73.6) | 429 (91.9) | 0.08 | 0.24 | 0.03 |
| ŌĆāŌĆāŌĆāInterstitial inflammation | 49 (77.8) | 88 (85.4) | 58 (63.7) | 424 (90.8) | 0.21 | 0.08 | 0.004 |
| ŌĆāŌĆāVessel | |||||||
| ŌĆāŌĆāŌĆāArteriosclerosis | 19 (30.2) | 34 (33.0) | 4 (4.4) | 92 (19.7) | 0.73 | <0.001 | 0.07 |
| ŌĆāŌĆāŌĆāIntimal thickening | 28 (44.4) | 62 (60.2) | 33 (36.3) | 194 (41.5) | 0.06 | 0.32 | 0.67 |
| ŌĆāIF staining on glomeruli (intensity) | |||||||
| ŌĆāŌĆāŌĆāIgG | 0.06 ┬▒ 0.23 | 0.07 ┬▒ 0.23 | 0.10 ┬▒ 0.27 | 0.63 ┬▒ 0.74 | 0.80 | 0.33 | <0.001 |
| ŌĆāŌĆāŌĆāIgM | 1.24 ┬▒ 0.50 | 0.23 ┬▒ 0.36 | 0.24 ┬▒ 0.34 | 0.86 ┬▒ 0.67 | <0.001 | <0.001 | <0.001 |
| ŌĆāŌĆāŌĆāIgA | 0.15 ┬▒ 0.36 | 0.16 ┬▒ 0.39 | 0.14 ┬▒ 0.30 | 2.64 ┬▒ 0.60 | 0.94 | 0.88 | <0.001 |
| ŌĆāŌĆāŌĆāC3 | 0.44 ┬▒ 0.74 | 0.14 ┬▒ 0.33 | 0.08 ┬▒ 0.21 | 1.86 ┬▒ 0.89 | 0.002 | <0.001 | <0.001 |
| ŌĆāŌĆāŌĆāC1q | 0.48 ┬▒ 0.72 | 0.12 ┬▒ 0.36 | 0.14 ┬▒ 0.25 | 0.23 ┬▒ 0.43 | <0.001 | <0.001 | 0.008 |
| ŌĆāŌĆāŌĆāFibrinogen | 0.40 ┬▒ 0.26 | 0.02 ┬▒ 0.15 | 0.01 ┬▒ 0.07 | 0.12 ┬▒ 0.44 | 0.63 | 0.40 | 0.03 |
| ŌĆāŌĆāŌĆāKappa chain | 0.08 ┬▒ 0.31 | 0.04 ┬▒ 0.15 | 0.02 ┬▒ 0.09 | 0.52 ┬▒ 0.88 | 0.34 | 0.13 | <0.001 |
| ŌĆāŌĆāŌĆāLambda chain | 0.09 ┬▒ 0.32 | 0.06 ┬▒ 0.21 | 0.06 ┬▒ 0.18 | 0.84 ┬▒ 1.08 | 0.55 | 0.51 | <0.001 |
| ŌĆāIF staining on glomeruli (n Ōēź 1+, %) | |||||||
| ŌĆāŌĆāŌĆāIgG | 3 (4.8) | 4 (3.9) | 6 (6.6) | 208 (44.5) | >0.99 | 0.74 | <0.001 |
| ŌĆāŌĆāŌĆāIgM | 63 (100) | 13 (12.6) | 10 (11.0) | 279 (59.7) | <0.001 | <0.001 | <0.001 |
| ŌĆāŌĆāŌĆāIgA | 4 (6.3) | 10 (9.7) | 7 (7.7) | 459 (98.3) | 0.57 | >0.99 | <0.001 |
| ŌĆāŌĆāŌĆāC3 | 15 (23.8) | 7 (6.8) | 2 (2.2) | 417 (88.3) | 0.004 | <0.001 | <0.001 |
| ŌĆāŌĆāŌĆāC1q | 20 (31.7) | 7 (6.8) | 2 (2.2) | 62 (13.3) | <0.001 | <0.001 | 0.001 |
| ŌĆāŌĆāŌĆāFibrinogen | 1 (1.6) | 2 (1.9) | 0 (0) | 36 (7.7) | >0.99 | 0.41 | 0.11 |
| ŌĆāŌĆāŌĆāKappa chain | 3 (4.8) | 1 (1.0) | 0 (0) | 124 (26.6) | 0.15 | 0.07 | <0.001 |
| ŌĆāŌĆāŌĆāLambda chain | 3 (4.8) | 3 (2.9) | 1 (1.1) | 190 (40.7) | 0.67 | 0.31 | <0.001 |
| ŌĆāElectron microscopic findings | |||||||
| ŌĆāŌĆāElectron-dense deposit | |||||||
| ŌĆāŌĆāŌĆāMesangial | 13 (20.6) | 0 (0) | 0 (0) | 427 (91.0) | <0.001 | <0.001 | <0.001 |
| ŌĆāŌĆāŌĆāSubepithelial space | 3 (4.8) | 0 (0) | 0 (0) | 2 (0.4) | 0.05 | 0.004 | 0.15 |
| ŌĆāŌĆāŌĆāSubendothelial space | 6 (9.5) | 0 (0) | 0 (0) | 24 (5.1) | 0.003 | 0.07 | 0.01 |
| ŌĆāPodocyte foot process | |||||||
| ŌĆāŌĆāDiffuse effacement | 36 (57.1) | 68 (66.0) | 74 (81.3) | 151 (32.2) | 0.32 | 0.002 | <0.001 |
Data are expressed as number only, mean ┬▒ standard deviation, or number (%).
AKI, acute kidney injury based on the lowest creatinine value during the follow-up period; CHD, coronary heart disease; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate by CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation; HPF, high-power field; FSGS, focal segmental glomerulosclerosis; IF, immunofluorescent; Ig, immunoglobulin; IgAN, IgA nephropathy; IgMN, IgM nephropathy; MCD, minimal change disease; RBC, red blood cell; SBP, systolic blood pressure; UPCR, spot urine protein-to-creatinine ratio.
Table┬Ā4.
| Pathologic diagnosis | IgMN (n = 63) | FSGS (n = 103) | MCD (n = 91) | IgAN (n = 469) | p-value* | p-value** | p-value*** |
|---|---|---|---|---|---|---|---|
| FU after biopsya (mo) | 76.6 ┬▒ 64.0 | 73.4 ┬▒ 56.5 | 55.7 ┬▒ 49.5 | 82.2 ┬▒ 54.4 | 0.73 | 0.03 | 0.51 |
| No. of laboratory tests during FU (/mo) | 1.7 ┬▒ 3.0 | 1.3 ┬▒ 3.2 | 4.8 ┬▒ 15.7 | 0.9 ┬▒ 7.0 | 0.39 | 0.07 | 0.35 |
| UPCR at last visit (g/g Cr) | 2.40 ┬▒ 5.02 | 1.93 ┬▒ 3.48 | 1.15 ┬▒ 3.73 | 0.95 ┬▒ 1.54 | 0.49 | 0.08 | |
| ŌĆā<0.30 | 19 (31.1) | 28 (27.2) | 65 (73.0) | 185 (39.4) | 0.28 | <0.001 | <0.001 |
| ŌĆā0.30ŌĆō2.99 | 29 (47.5) | 61 (59.2) | 16 (18.0) | 253 (53.9) | |||
| ŌĆāŌēź3.00 g/g Cr | 13 (21.3) | 14 (13.6) | 8 (9.0) | 31 (6.6) | |||
| Creatinine at last visit (mg/dL) | 2.69 ┬▒ 3.37 | 2.28 ┬▒ 2.41 | 1.00 ┬▒ 0.63 | 1.67 ┬▒ 2.07 | 0.37 | <0.001 | 0.02 |
| eGFR at last visit (mL/min/1.73 m2) | 58 ┬▒ 38 | 58 ┬▒ 39 | 89 ┬▒ 30 | 73 ┬▒ 35 | 0.97 | <0.001 | 0.001 |
| Treatment after biopsy | |||||||
| ŌĆāRAS blocker | 55 (87.3) | 88 (85.4) | 45 (49.5) | 405 (86.4) | 0.82 | <0.001 | >0.99 |
| ŌĆāAntihypertensive medication | 57 (90.5) | 93 (90.3) | 57 (62.6) | 420 (89.6) | >0.99 | <0.001 | >0.99 |
| ŌĆāAntidiabetic medication | 24 (38.1) | 28 (27.2) | 28 (30.8) | 58 (12.4) | 0.17 | 0.39 | <0.001 |
| Immunosuppressive treatment after biopsy | 36 (57.1) | 44 (42.7) | 63 (69.2) | 157 (33.5) | 0.08 | 0.13 | <0.001 |
| ŌĆāSteroid only | 17 (27.0) | 24 (23.3) | 31 (34.1) | 85 (18.1) | |||
| ŌĆāSteroid and cyclophosphamide | 8 (12.7) | 6 (5.8) | 8 (8.8) | 37 (7.9) | 0.27 | 0.27 | 0.003 |
| ŌĆāSteroids and other immunosuppressantsb | 11 (17.5) | 13 (12.6) | 24 (26.4) | 33 (7.0) | |||
| ŌĆāMedications other than cyclophosphamide and steroid | 0 (0) | 1 (1.0) | 0 (0) | 2 (0.4) | |||
| Outcomes | |||||||
| ŌĆāRenal eventcduring FU | 29 (46.0) | 42 (40.8) | 17 (18.7) | 124 (26.4) | 0.52 | <0.001 | 0.001 |
| ŌĆāESRD during FU | 12 (20.0) | 16 (15.7) | 10 (11.0) | 37 (7.9) | 0.52 | 0.16 | 0.007 |
| ŌĆāDeath during FU | 5 (7.9) | 6 (5.8) | 3 (3.3) | 3 (0.6) | 0.75 | 0.27 | 0.002 |
Data are expressed as mean ┬▒ standard deviation or number (%).
ESRD, end-stage renal disease; eGFR, estimated glomerular filtration rate; FSGS, focal segmental glomerulosclerosis; FU, follow-up; Ig, immunoglobulin; IgAN, IgA nephropathy; IgMN, IgM nephropathy; MCD, minimal change disease; RAS, renin-angiotensin-aldosterone; UPCR, urine protein-to-creatinine ratio.
References
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 489 View
- 21 Download
- ORCID iDs
-
Giae Yun

https://orcid.org/0000-0003-3712-9011Eun Jeong Kwon

https://orcid.org/0000-0003-4225-0270Seokwoo Park

https://orcid.org/0000-0003-2758-1362Jong Cheol Jeong

https://orcid.org/0000-0003-0301-7644Sejoong Kim

https://orcid.org/0000-0002-7238-9962Ki Young Na

https://orcid.org/0000-0002-8872-8236Ho Jun Chin

https://orcid.org/0000-0002-3710-0190 - Related articles
-
Clinicopathologic Characteristics of IgA Nephropathy with Crescents2011 March;30(2)
Plasma endocan level and prognosis of immunoglobulin A nephropathy2016 September;35(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print















