| Kidney Res Clin Pract > Volume 43(2); 2024 > Article |
|
Abstract
Background
Methods
Results
Supplementary Materials
Notes
Funding
This research was supported by a grant funded by Seoul National University Hospital (grant number: 3020-210020). This research was supported by a grant from the MD-PhD/Medical Scientist Training Program through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea. This study was supported by the 2022 Young Investigator Research Grant from the Korean Nephrology Research Foundation.
Data sharing statement
The data presented in this study are available upon reasonable request from the corresponding author.
Authors’ contributions
Conceptualization, Supervision: DKK, KH, SP
Data curation, Formal analysis, Methodology, Resources: KH
Investigation: SL, YK, SC, HH, DKK, KH
Project administration: DKK, SP
Validation: SL, YK, SC, HH, YCK, SSH, HL, JPL, KWJ, CSL, YSK, DKK
Writing–original draft: SJ
Writing–review & editing: SP
All authors read and approved the final manuscript.
Acknowledgments
Figure 2.
The incidence rate of death by cause.
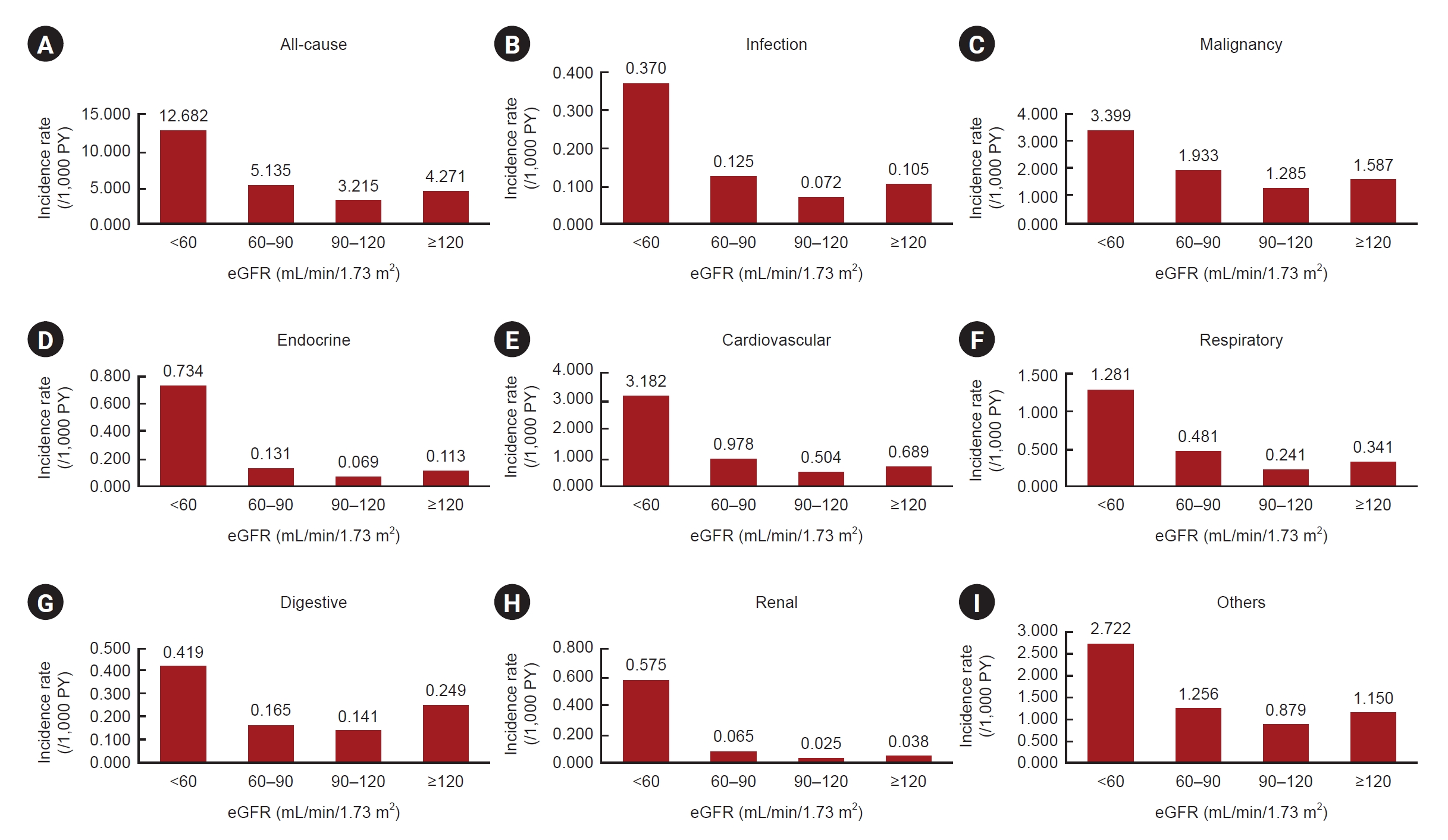
Figure 3.
Nonlinear associations in the multivariable analysis between eGFR and causes of death.

Figure 4.
Risk of death by all-cause mortality according to baseline eGFR and albuminuria.
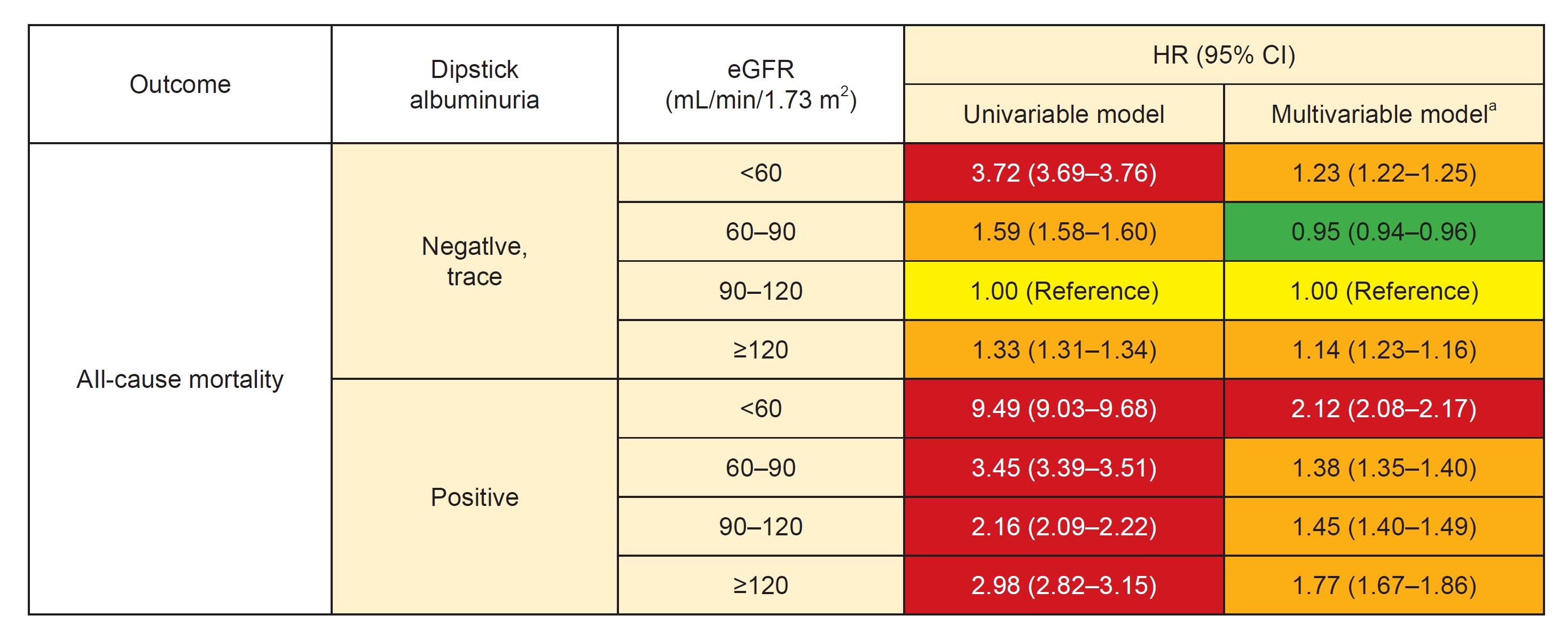
Table 1.
| Variable |
eGFR (mL/min/1.73 m2) |
|||
|---|---|---|---|---|
| <60 (n = 684,485) | 60–90 (n = 5,318,506) | 90–120 (n = 3,245,608) | ≥120 (n = 669,239) | |
| Age (yr) | 55.37 ± 15.74 | 48.82 ± 13.68 | 43.86 ± 12.96 | 42.80 ± 14.52 |
| Male sex | 330,305 (48.3) | 2,964,619 (55.7) | 1,768,223 (54.5) | 357,571 (53.4) |
| Body shape measures | ||||
| Height (cm) | 161.53 ± 9.73 | 163.82 ± 9.25 | 164.30 ± 9.07 | 164.08 ± 9.01 |
| Weight (kg) | 63.02 ± 11.25 | 64.31 ± 11.54 | 63.60 ± 11.74 | 62.61 ± 11.99 |
| Body mass index (kg/m2) | 24.07 ± 3.22 | 23.87 ± 3.34 | 23.47 ± 3.66 | 23.16 ± 3.39 |
| Weight circumference (cm) | 81.84 ± 9.36 | 80.72 ± 9.36 | 79.40 ± 9.47 | 78.92 ± 9.92 |
| Social and lifestyle factors | ||||
| Smoking history | ||||
| Nonsmoker | 448,677 (65.6) | 3,156,232 (59.3) | 1,903,600 (58.7) | 396,622 (59.3) |
| Ex-smoker | 106,769 (15.6) | 820,191 (15.4) | 418,109 (12.9) | 81,093 (12.1) |
| Current-smoker | 129,039 (18.9) | 1,342,083 (25.2) | 923,899 (28.5) | 191,524 (28.6) |
| Drinkera | ||||
| Nondrinker | 421,893 (61.6) | 2,797,673 (52.6) | 1,583,268 (48.8) | 322,519 (48.2) |
| Mild drinker | 225,684 (33.0) | 2,115,553 (39.8) | 1,378,987 (42.5) | 282,302 (42.2) |
| Heavy drinker | 36,908 (5.4) | 405,280 (7.6) | 283,353 (8.7) | 64,418 (9.6) |
| Regular physical activityb | 133,339 (19.5) | 1,001,108 (18.8) | 544,318 (16.8) | 101,900 (15.2) |
| Low incomec | 116,014 (17.0) | 1,012,050 (19.0) | 656,962 (20.2) | 146,877 (22.0) |
| Baseline comorbidities | ||||
| Diabetes mellitus | 109,829 (16.1) | 474,051 (8.9) | 235,131 (7.2) | 54,636 (8.2) |
| Hypertension | 292,029 (42.7) | 1,463,229 (27.5) | 677,855 (20.9) | 142,732 (21.3) |
| Hyperlipidemia | 192,190 (28.1) | 1,043,428 (19.6) | 473,998 (14.6) | 90,963 (13.6) |
| Laboratory measurements | ||||
| Systolic blood pressure (mmHg) | 125.39 ± 16.01 | 122.88 ± 15.09 | 121.45 ± 14.73 | 121.29 ± 15.05 |
| Diastolic blood pressure (mmHg) | 77.19 ± 10.19 | 76.64 ± 10.03 | 75.82 ± 10.04 | 75.40 ± 10.24 |
| Impaired fasting glucose (mg/dL) | 102.00 ± 29.33 | 97.74 ± 23.5 | 96.01 ± 23.02 | 95.87 ± 24.99 |
| Hemoglobin (g/dL) | 13.67 ± 1.68 | 14.00 ± 1.59 | 13.95 ± 1.60 | 13.80 ± 1.60 |
| Cholesterol (mg/dL) | 198.98 ± 43.92 | 197.73 ± 41.32 | 191.99 ± 40.56 | 188.10 ± 42.56 |
| HDL (mg/dL) | 61.37 ± 66.82 | 55.41 ± 25.42 | 56.36 ± 28.42 | 60.20 ± 48.09 |
| LDL (mg/dL) | 118.64 ± 90.70 | 124.09 ± 224.17 | 119.89 ± 239.64 | 108.67 ± 108.34 |
| eGFR (mL/min/1.73 m2) | 37.27 ± 23.01 | 77.00 ± 7.65 | 100.75 ± 8.12 | 159.48 ± 139.62 |
| Urine protein | ||||
| Negative, trace | 643,468 (94.0) | 5,187,036 (97.5) | 3,181,300 (98.0) | 655,242 (97.9) |
| Positive | 41,017 (6.0) | 131,470 (2.5) | 64,308 (2.0) | 13,997 (2.1) |
Data are expressed as mean ± standard deviation or number (%).
eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
a Drinkers are categorized into three groups: nondrinkers (0 g/day), mild drinkers (0–30 g/day), and heavy drinkers (≥30 g/day).
Table 2.
| Causes of death | eGFR (mL/min/1.73 m2) | No. of specific diseases | No. of events | Incidence rate (/1,000 PY) |
Univariable model |
Multivariable modela |
||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||||
| All-cause | <60 | 684,485 | 85,597 | 12.682 | 3.95 (3.91–3.98) | <0.001 | 1.26 (1.24–1.27) | <0.001 |
| 60–90 | 5,318,506 | 277,125 | 5.135 | 1.60 (1.59–1.61) | <0.001 | 0.95 (0.94–0.96) | <0.001 | |
| 90–120 | 3,245,608 | 106,531 | 3.215 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 669,239 | 29,000 | 4.271 | 1.33 (1.31–1.35) | <0.001 | 1.15 (1.13–1.16) | <0.001 | |
| Infection | <60 | 684,485 | 2,499 | 0.370 | 5.17 (4.88–5.46) | <0.001 | 1.32 (1.25–1.40) | <0.001 |
| 60–90 | 5,318,506 | 6,731 | 0.125 | 1.74 (1.66–1.82) | <0.001 | 0.94 (0.89–0.98) | 0.008 | |
| 90–120 | 3,245,608 | 2,377 | 0.072 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 669,239 | 711 | 0.105 | 1.46 (1.35–1.59) | <0.001 | 1.19 (1.10–1.30) | <0.001 | |
| Malignancy | <60 | 684,485 | 22,941 | 3.399 | 2.65 (2.61–2.69) | <0.001 | 0.95 (0.94–0.97) | <0.001 |
| 60–90 | 5,318,506 | 104,304 | 1.933 | 1.50 (1.49–1.52) | <0.001 | 0.94 (0.93–0.95) | <0.001 | |
| 90–120 | 3,245,608 | 42,574 | 1.285 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 669,239 | 10,773 | 1.587 | 1.24 (1.21–1.26) | <0.001 | 1.10 (1.08–1.12) | <0.001 | |
| Endocrine | <60 | 684,485 | 4,952 | 0.734 | 10.65 (10.14–11.19) | <0.001 | 2.10 (1.99–2.21) | <0.001 |
| 60–90 | 5,318,506 | 7,086 | 0.131 | 1.90 (1.82–2.00) | <0.001 | 1.02 (0.97–1.07) | 0.43 | |
| 90–120 | 3,245,608 | 2,286 | 0.069 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 669,239 | 765 | 0.113 | 1.64 (1.51–1.77) | <0.001 | 1.29 (1.19–1.40) | <0.001 | |
| Cardiovascular | <60 | 684,485 | 21,479 | 3.182 | 6.32 (6.19–6.45) | <0.001 | 1.54 (1.51–1.57) | <0.001 |
| 60–90 | 5,318,506 | 52,763 | 0.978 | 1.94 (1.91–1.97) | <0.001 | 1.03 (1.01–1.05) | 0.003 | |
| 90–120 | 3,245,608 | 16,707 | 0.504 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 669,239 | 4,681 | 0.689 | 1.37 (1.33–1.41) | <0.001 | 1.14 (1.10–1.17) | <0.001 | |
| Respiratory | <60 | 684,485 | 8,647 | 1.281 | 5.33 (5.17–5.50) | <0.001 | 1.19 (1.16–1.23) | <0.001 |
| 60–90 | 5,318,506 | 25,986 | 0.481 | 2.00 (1.95–2.05) | <0.001 | 0.97 (0.95–1.00) | 0.03 | |
| 90–120 | 3,245,608 | 7,975 | 0.241 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 669,239 | 2,316 | 0.341 | 1.42 (1.36–1.49) | <0.001 | 1.14 (1.09–1.20) | <0.001 | |
| Digestive | <60 | 684,485 | 2,827 | 0.419 | 2.98 (2.85–3.13) | <0.001 | 1.21 (1.15–1.27) | <0.001 |
| 60–90 | 5,318,506 | 8,920 | 0.165 | 1.16 (1.14–1.218) | <0.001 | 0.82 (0.78–0.84) | <0.001 | |
| 90–120 | 3,245,608 | 4,657 | 0.141 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 669,239 | 1,693 | 0.249 | 1.78 (1.68–1.88) | <0.001 | 1.51 (1.43–1.60) | <0.001 | |
| Renal | <60 | 684,485 | 3,878 | 0.575 | 22.77 (21.13–24.53) | <0.001 | 4.09 (3.78–4.42) | <0.001 |
| 60–90 | 5,318,506 | 3,526 | 0.065 | 2.58 (2.39–2.78) | <0.001 | 1.23 (1.14–1.33) | <0.001 | |
| 90–120 | 3,245,608 | 839 | 0.025 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 669,239 | 256 | 0.038 | 1.50 (1.30–1.72) | <0.001 | 1.19 (1.04–1.37) | 0.01 | |
| Others | <60 | 684,485 | 18,374 | 2.722 | 3.10 (3.04–3.16) | <0.001 | 1.22 (1.20–1.25) | <0.001 |
| 60–90 | 5,318,506 | 67,809 | 1.256 | 1.43 (1.41–1.45) | <0.001 | 0.94 (0.92–0.95) | <0.001 | |
| 90–120 | 3,245,608 | 29,116 | 0.879 | 1.00 (Reference) | 1.00 (Reference) | |||
| ≥120 | 1.31 (1.28–1.34) | <0.001 | 1.16 (1.13–1.19) | <0.001 | ||||
Table 3.
| Type of death | Dipstick | No. of specific diseases | No. of events | Incidence rate (/1,000 PY) |
Univariable model |
Multivariable modela |
||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | p-value | HR (95% CI) | p-value | |||||
| Overall | Negative, trace | 9,667,046 | 467,571 | 4.762 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 17,427 | 10.415 | 2.20 (2.16–2.23) | <0.001 | 1.40 (1.38–1.42) | <0.001 | |
| 2+ | 62,608 | 9,295 | 15.329 | 3.24 (3.18–3.31) | <0.001 | 1.77 (1.74–1.81) | <0.001 | |
| 3, 4+ | 19,115 | 3,960 | 22.117 | 4.71 (4.57–4.86) | <0.001 | 2.29 (2.22–2.36) | <0.001 | |
| Infection | Negative, trace | 9667,046 | 11,560 | 0.118 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 434 | 0.259 | 2.21 (2.01–2.44) | <0.001 | 1.38 (1.25–1.52) | <0.001 | |
| 2+ | 62,608 | 218 | 0.360 | 3.08 (2.70–3.524) | <0.001 | 1.65 (1.44–1.89) | <0.001 | |
| 3, 4+ | 19,115 | 106 | 0.592 | 5.14 (4.24–6.2) | <0.001 | 2.44 (2.02–2.96) | <0.001 | |
| Malignancy | Negative, trace | 9,667,046 | 171,933 | 1.751 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 5,372 | 3.211 | 1.84 (1.79–1.89) | <0.001 | 1.25 (1.22–1.29) | <0.001 | |
| 2+ | 62,608 | 2,432 | 4.011 | 2.30 (2.21–2.40) | <0.001 | 1.38 (1.33–1.44) | <0.001 | |
| 3, 4+ | 19,115 | 855 | 4.775 | 2.76 (2.58–2.95) | <0.001 | 1.51 (1.41–1.61) | <0.001 | |
| Endocrine | Negative, trace | 9,667,046 | 12,422 | 0.127 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 1,102 | 0.659 | 5.23 (4.92–5.56) | <0.001 | 1.94 (1.82–2.06) | <0.001 | |
| 2+ | 62,608 | 985 | 1.624 | 12.94 (12.13–13.81) | <0.001 | 3.40 (3.18–3.63) | <0.001 | |
| 3, 4+ | 19,115 | 580 | 3.239 | 25.96 (23.88–28.21) | <0.001 | 5.16 (4.74–5.62) | <0.001 | |
| Cardiovascular | Negative, trace | 9,667,046 | 88,491 | 0.901 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 4,008 | 2.395 | 2.67 (2.59–2.76) | <0.001 | 1.55 (1.50–1.60) | <0.001 | |
| 2+ | 62,608 | 2,175 | 3.587 | 4.01 (3.85–4.19) | <0.001 | 1.94 (1.86–2.02) | <0.001 | |
| 3, 4+ | 19,115 | 956 | 5.339 | 6.01 (5.64–6.41) | <0.001 | 2.50 (2.34–2.66) | <0.001 | |
| Respiratory | Negative, trace | 9,667,046 | 42,375 | 0.432 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 1,554 | 0.929 | 2.17 (2.06–2.28) | <0.001 | 1.37 (1.30–1.44) | <0.001 | |
| 2+ | 62,608 | 712 | 1.174 | 2.76 (2.56–2.97) | <0.001 | 1.54 (1.43–1.66) | <0.001 | |
| 3, 4+ | 19,115 | 283 | 1.581 | 3.76 (3.35–4.23) | <0.001 | 1.88 (1.67–2.11) | <0.001 | |
| Digestive | Negative, trace | 9,667,046 | 16,869 | 0.172 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 684 | 0.409 | 2.39 (2.21–2.58) | <0.001 | 1.46 (1.35–1.58) | <0.001 | |
| 2+ | 62,608 | 388 | 0.640 | 3.75 (3.39–4.15) | <0.001 | 1.94 (1.75–2.15) | <0.001 | |
| 3, 4+ | 19,115 | 156 | 0.871 | 5.15 (4.4–6.03) | <0.001 | 2.37 (2.02–2.78) | <0.001 | |
| Renal | Negative, trace | 9,667,046 | 6,649 | 0.068 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 731 | 0.437 | 6.51 (6.03–7.03) | <0.001 | 2.99 (2.77–3.23) | <0.001 | |
| 2+ | 62,608 | 707 | 1.166 | 17.49 (16.19–18.90) | <0.001 | 6.00 (5.54–6.49) | <0.001 | |
| 3, 4+ | 19,115 | 412 | 2.301 | 34.99 (31.67–38.65) | <0.001 | 9.60 (8.67–10.64) | <0.001 | |
| Others | Negative, trace | 9,667,046 | 117,272 | 1.194 | 1.00 (Reference) | 1.00 (Reference) | ||
| 1+ | 169,069 | 3,542 | 2.117 | 1.78 (1.72–1.84) | <0.001 | 1.24 (1.19–1.28) | <0.001 | |
| 2+ | 62,608 | 1,678 | 2.767 | 2.33 (2.22–2.45) | <0.001 | 1.43 (1.36–1.50) | <0.001 | |
| 3, 4+ | 19,115 | 612 | 3.418 | 2.89 (2.67–3.13) | <0.001 | 1.61 (1.48–1.74) | <0.001 | |
Table 4.
| Type of death | Dipstick albuminuria | eGFR (mL/min/1.73 m2) | HR (95% CI) | |
|---|---|---|---|---|
|
Univariable model |
Multivariable modela |
|||
| Infection | Negative, trace | <60 | 4.94 (4.66–5.24) | 1.31 (1.23–1.39) |
| 60–90 | 1.73 (1.65–1.81) | 0.94 (0.89–0.98) | ||
| 90–120 | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥120 | 1.46 (1.34–1.59) | 1.19 (1.09–1.30) | ||
| Positive | <60 | 11.18 (9.89–12.64) | 2.06 (1.82–2.34) | |
| 60–90 | 3.78 (3.37–4.23) | 1.36 (1.21–1.53) | ||
| 90–120 | 2.17 (1.77–2.66) | 1.44 (1.18–1.77) | ||
| ≥120 | 3.11 (2.17–4.46) | 1.77 (1.23–2.53) | ||
| Malignancy | Negative, trace | <60 | 2.59 (2.54–2.63) | 0.96 (0.94–0.97) |
| 60–90 | 1.50 (1.48–1.52) | 0.94 (0.93–0.95) | ||
| 90–120 | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥120 | 1.23 (1.21–1.26) | 1.10 (1.07–1.12) | ||
| Positive | <60 | 4.61 (4.41–4.81) | 1.20 (1.15–1.25) | |
| 60–90 | 2.79 (2.70–2.87) | 1.23 (1.19–1.27) | ||
| 90–120 | 1.89 (1.80–1.99) | 1.35 (1.29–1.42) | ||
| ≥120 | 2.38 (2.16–2.63) | 1.54 (1.40–1.70) | ||
| Endocrine | Negative, trace | <60 | 8.89 (8.42–9.39) | 1.97 (1.86–2.09) |
| 60–90 | 1.88 (1.78–1.97) | 1.02 (0.97–1.07) | ||
| 90–120 | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥120 | 1.64 (1.50–1.79) | 1.30 (1.19–1.41) | ||
| Positive | <60 | 61.53 (57.49–65.85) | 6.02 (5.61–6.46) | |
| 60–90 | 11.30 (10.45–12.21) | 2.37 (2.19–2.57) | ||
| 90–120 | 6.36 (5.59–7.25) | 2.25 (1.98–2.57) | ||
| ≥120 | 9.88 (7.95–12.28) | 2.85 (2.30–3.54) | ||
| Cardiovascular | Negative, trace | <60 | 6.00 (5.87–6.12) | 1.54 (1.501–1.57) |
| 60–90 | 1.93 (1.89–1.96) | 1.03 (1.01–1.05) | ||
| 90–120 | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥120 | 1.38 (1.33–1.42) | 1.14 (1.10–1.18) | ||
| Positive | <60 | 15.85 (15.23–16.50) | 2.67 (2.57–2.78) | |
| 60–90 | 5.09 (4.90–5.29) | 1.70 (1.63–1.76) | ||
| 90–120 | 2.75 (2.57–2.94) | 1.70 (1.58–1.82) | ||
| ≥120 | 3.31 (2.90–3.78) | 1.79 (1.57–2.04) | ||
| Respiratory | Negative, trace | <60 | 5.17 (5.01–5.34) | 1.19 (1.16–1.23) |
| 60–90 | 1.99 (1.94–2.05) | 0.97 (0.95–1.00) | ||
| 90–120 | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥120 | 1.42 (1.36–1.49) | 1.14 (1.09–1.20) | ||
| Positive | <60 | 10.42 (9.72–11.17) | 1.72 (1.61–1.85) | |
| 60–90 | 4.09 (3.85–4.34) | 1.37 (1.29–1.45) | ||
| 90–120 | 2.15 (1.92–2.40) | 1.45 (1.29–1.62) | ||
| ≥120 | 2.98 (2.43–3.64) | 1.80 (1.47–2.20) | ||
| Digestive | Negative, trace | <60 | 2.85 (2.71–3.00) | 1.22 (1.16–1.28) |
| 60–90 | 1.17 (1.13–1.21) | 0.81 (0.78–0.84) | ||
| 90–120 | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥120 | 1.75 (1.66–1.86) | 1.49 (1.41–1.58) | ||
| Positive | <60 | 7.06 (6.34–7.87) | 1.87 (1.67–2.08) | |
| 60–90 | 3.01 (2.75–3.29) | 1.29 (1.18–1.41) | ||
| 90–120 | 2.69 (2.36–3.07) | 1.65 (1.45–1.88) | ||
| ≥120 | 5.71 (4.71–6.91) | 2.99 (2.47–3.63) | ||
| Renal | Negative, trace | <60 | 17.55 (16.18–19.03) | 3.69 (3.39–4.01) |
| 60–90 | 2.52 (2.33–2.73) | 1.23 (1.14–1.33) | ||
| 90–120 | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥120 | 1.49 (1.29–1.73) | 1.19 (1.02–1.37) | ||
| Positive | <60 | 154.38 (141.11–168.91) | 19.79 (18.02–21.73) | |
| 60–90 | 14.99 (13.34–16.85) | 4.11 (3.66–4.63) | ||
| 90–120 | 5.78 (4.62–7.22) | 3.14 (2.51–3.93) | ||
| ≥120 | 8.52 (5.80–12.50) | 3.94 (2.68–5.78) | ||
| Others | Negative, trace | <60 | 3.01 (2.95–3.06) | 1.22 (1.20–1.24) |
| 60–90 | 1.42 (1.40–1.44) | 0.94 (0.92–0.95) | ||
| 90–120 | 1.00 (Reference) | 1.00 (Reference) | ||
| ≥120 | 1.30 (1.27–1.34) | 1.15 (1.13–1.18) | ||
| Positive | <60 | 5.49 (5.23–5.76) | 1.60 (1.52–1.68) | |
| 60–90 | 2.49 (2.40–2.59) | 1.18 (1.14–1.23) | ||
| 90–120 | 1.73 (1.62–1.84) | 1.26 (1.18–1.35) | ||
| ≥120 | 2.59 (2.31–2.89) | 1.68 (1.51–1.88) | ||
References
- TOOLS
-
METRICS

-
- 0 Crossref
- 0 Scopus
- 757 View
- 57 Download
- ORCID iDs
-
Sehyun Jung

https://orcid.org/0000-0002-2510-8822Soojin Lee

https://orcid.org/0000-0001-5633-3961Yaerim Kim

https://orcid.org/0000-0003-1596-1528Semin Cho

https://orcid.org/0000-0002-6060-9032Hyuk Huh

https://orcid.org/0000-0001-7608-0199Yong Chul Kim

https://orcid.org/0000-0003-3215-8681Seung Seok Han

https://orcid.org/0000-0003-0137-5261Hajeong Lee

https://orcid.org/0000-0002-1873-1587Jung Pyo Lee

https://orcid.org/0000-0002-4714-1260Kwon Wook Joo

https://orcid.org/0000-0001-9941-7858Chun Soo Lim

https://orcid.org/0000-0001-9123-6542Yon Su Kim

https://orcid.org/0000-0003-3091-2388Dong Ki Kim

https://orcid.org/0000-0002-5195-7852Kyungdo Han

https://orcid.org/0000-0002-6096-1263Sehoon Park

https://orcid.org/0000-0002-4221-2453 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















