Introduction
Immunoglobulin A nephropathy (IgAN) is characterized by the deposition of circulating immune complexes containing undergalactosylated IgA in the glomerular mesangium [
1]. Several lines of evidence have shown the involvement of systemic immune dysregulation in the pathophysiology of IgAN. For instance, IgAN has a recurrence rate of 50% after renal transplantation [
2]. Moreover, IgAN resolved after bone marrow transplantation in a patient with IgAN and chronic myeloblastic leukemia [
3].
The imbalance in T helper (Th) cells, which are involved in adaptive immune responses, has been suggested to play an important role in the pathogenesis of several diseases [
4], including IgAN. Representative Th1/Th2 cytokines, interferon-gamma (IFN╬│), and interleukin (IL)-4 were found to be involved in the pathogenesis, development, and progression of IgAN [
5-
7]. The Th1/Th2 cytokine polarity has been shown to play a key role in these processes; however, the results of these studies have not been consistent. Furthermore, in previous studies [
5-
7], the cytokine profile of specific specimens, such as serum, urine, kidney, or tonsil, was determined, but a global understanding of the cytokine landscape is currently lacking.
To address the inconsistency of previous results, we evaluated the levels of Th1/Th2 cytokines in various samples, including serum, urine, kidney, peripheral blood mononuclear cells (PBMCs), and mesangial cells (MCs), from patients with IgAN. These measurements may more clearly reveal the relationships of cytokines with clinical parameters. We also evaluated the correlation of cytokine levels and the response to glucocorticoid treatment in patients with IgAN.
Methods
Participants
Thirty-one patients with biopsy-proven IgAN and 25 healthy controls from three different hospitals were enrolled in this study. Patients with the following systemic diseases were excluded: autoimmune diseases, diabetes, hepatic diseases, and malignancies. IgAN was diagnosed based on the following pathologic findings; presence of mesangial proliferation assessed by light microscopy and mesangial IgA deposition assessed by immunofluorescence. All patients were treated with renin-angiotensin system blockade. Patients with proteinuria of >500 mg/day were treated with the following glucocorticoid regimen; 1 mg/kg/day at the beginning for 2 months and tapering for another 4 months. A 24-hour urine sample was collected to measure proteinuria. Blood and urine (for the measurement of cytokines) were immediately processed. Samples were centrifuged at 3,000 ├Ś g for 10 minutes, and aliquots were stored at -80┬░C until analysis. This study was approved by the Institutional Review Board of the Korea University (No. R0803121). Informed consent was obtained from all participants in compliance with the Helsinki Declaration.
Data collection
We collected data on patient demographics and comorbidities. The baseline laboratory examination included serum creatinine, cholesterol, high-density cholesterol, triglycerides, fasting blood glucose, hemoglobin, white blood cell count, platelets, and urine analysis.
Cytokine measurement
The simultaneous assessment of the concentration of IFN╬│, IL-2, IL-4, IL-10, IL-13, monocyte chemoattractant peptide (MCP)-1, and tumor necrosis factor alpha (TNF-╬▒) in the serum and urine was performed using commercially available multiplex bead-based sandwich-based immunoassay kits (MPXHCYTO-60K-07; Millipore, Billerica, MA, USA) following the manufacturerŌĆÖs instructions. The concentration of urinary cytokines was standardized against that of urinary creatinine. The assays were performed in duplicate. Secreted cytokines in culture supernatants were also measured using the same method. The levels of all cytokines are expressed relative to the total protein concentration.
Quantitative reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA from renal core biopsy tissues or cultured MCs were extracted using Trizol reagent (ThermoFisher Scientific Inc., Waltham, MA, USA) and reverse transcribed into cDNA using SuperScript II Reverse Transcriptase (Invitrogen, Carlsbad, CA, USA) as described elsewhere [
8]. Quantitative RT-PCR was performed on a Bio-Rad iCycler system (Bio-Rad, Hercules, CA, USA) using SYBR Green (TaKaRa Bio, Tokyo, Japan) using the following cycling conditions; 50┬░C for 10 minutes, 95┬░C for 5 minutes, and then 45 cycles of denaturation at 95┬░C for 10 seconds and annealing with extension at 60┬░C for 30 seconds. The sequences of primers used in this study are shown in
Supplementary Table 1 (available online). The expression of each gene was normalized to that of ╬▓-actin messenger RNA (mRNA).
Peripheral blood mononuclear cells
PBMCs were obtained using a Ficoll solution (Biochrom AG, Berlin, Germany) and resuspended in DulbeccoŌĆÖs modified Eagle medium (Gibco BRL, Grand Island, NY, USA) supplemented with 5% fetal calf serum at 37┬░C for 2 hours. Nonspecific esterase staining was performed to confirm the identity of PBMCs. For PBMC activation, cells were treated with phorbitol 12-myristate 13-acetate (Sigma Aldrich Corp., St. Louis, MO, USA) at a final dose of 25 ng/mL. After PBMCs were incubated for 48 hours, supernatants were collected and frozen at -80┬░C for subsequent experiments.
Mesangial cell culture
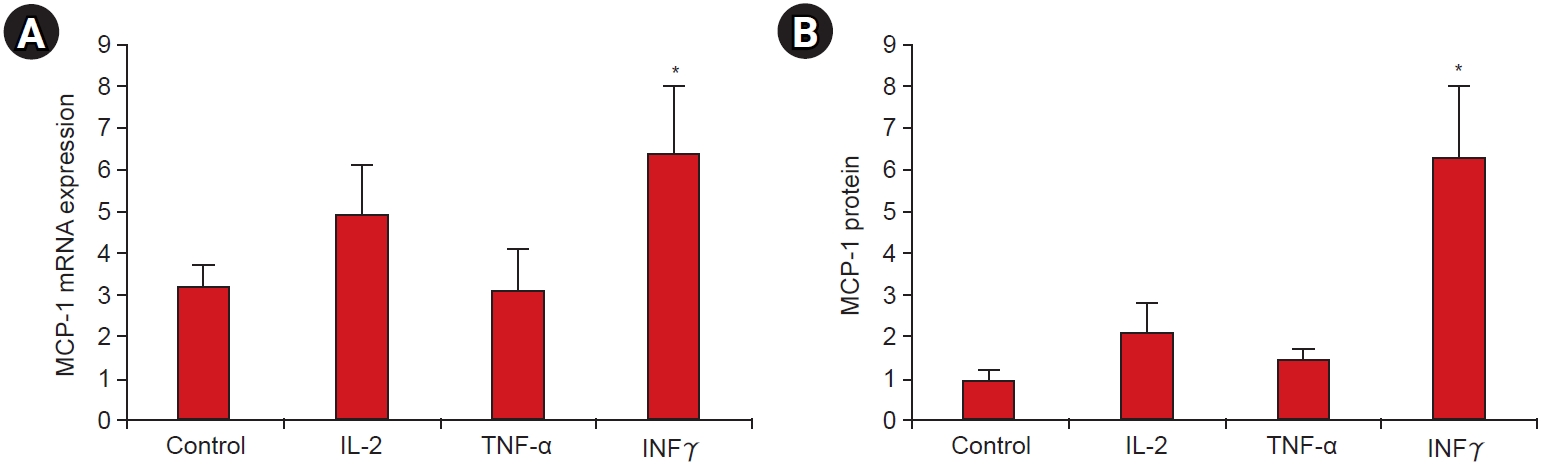
To access the production of cytokines by MCs, the normal renal cortex of human kidneys was obtained immediately after nephrectomy as previously described [
9]. To test the response of MCs to Th1/Th2 cytokines, MCs were incubated in medium containing IL-2 (10 ng/mL), TNF-╬▒ (25 ng/mL), or IFN╬│ (1,000 IU/mL) for 24 hours. MCP-1 protein level was determined according to the manufacturerŌĆÖs recommendation (MPXHCYTO-60K-07;Millipore).
Statistical analysis
Statistical analyses were performed using the IBM SPSS Statistics version 25.0 (IBM Corp., Armonk, NY, USA). The statistical significance of differences was determined by ANOVA or the Student t-test. The nonparametric Kruskal-Wallis test was performed if assumption of normality of the data was not suitable. The correlation between cytokine gene expression and clinicopathological data was analyzed by the Spearman test. A p-value of <0.05 was regarded as significant. All data are presented as mean ┬▒ standard deviation.
Discussion
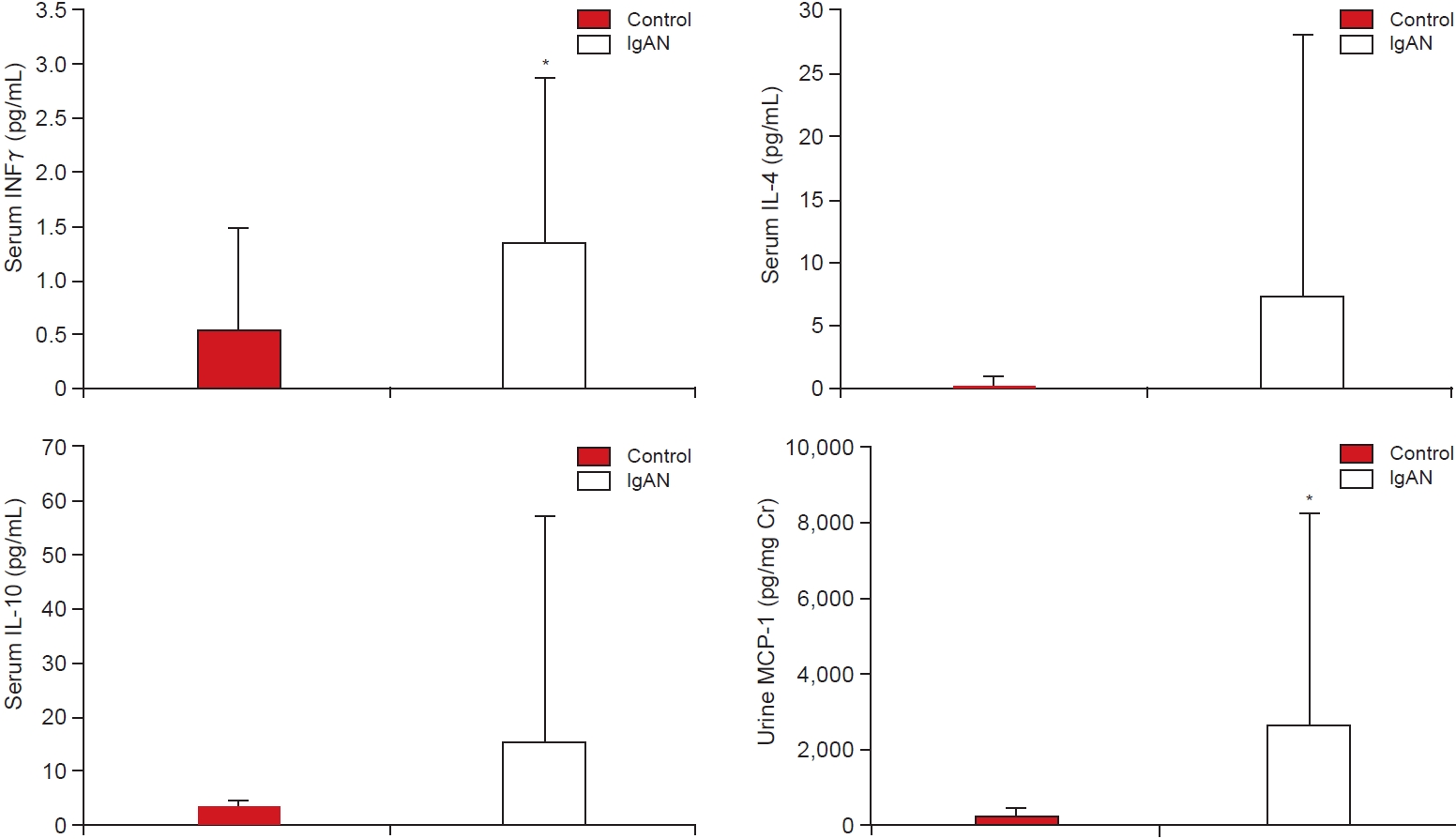
In this study, we found that levels of serum IFN╬│ and urinary MCP-1 were significantly higher in patients with IgAN compared with levels in healthy controls. Urinary MCP-1, serum IL-10, and TNF-╬▒ levels were higher in patients with high proteinuria or correlated with the proteinuria level. Furthermore, the urinary MCP-1 level was significantly increased in patients with low eGFR. In vitro experiments also showed that among different tested Th1/Th2 cytokines, only IFN╬│ induced MCP-1 synthesis in cultured patient-derived MCs. Therefore, IFN╬│ was found to be a key cytokine in the pathogenetic processes of IgAN; in turn, the upregulation of IFN╬│ induced an increase in urinary MCP-1 levels. Consequently, upregulated MCP-1 was related to clinical findings, such as proteinuria and eGFR levels, and further correlated with responsiveness to glucocorticoid treatment.
The importance of Th1/Th2 polarization in the pathogenesis of IgAN remains unclear. Some reports point to a Th1 predominance in patients with IgAN and animal models for IgAN [
5,
10,
11]. Moreover, the IgA-containing immune complex was found to induce the secretion of IL-1 and IL-6, subsequently leading to inflammatory reactions [
12]. Other studies showed that IFN╬│, TNF-╬▒, and IL-6 increased the expression of Fc╬▒ receptors in MCs and the interaction of IgA with Fc╬▒ receptors induced the expression of NF-╬║B and MCP-1 [
13,
14]. INF╬│ polymorphisms were also related to the development of IgAN [
15]. These findings support a relationship between Th1 cytokines and proinflammatory cytokines. In our study, we could not demonstrate definitive polarization of Th1/Th2 cells, but the IFN╬│ levels were predominantly increased in the serum of patients with IgAN and IFN╬│ stimulated MCP-1 expression in MCs from IgAN patients. Therefore, IFN╬│ might be a key cytokine in the development of IgAN.
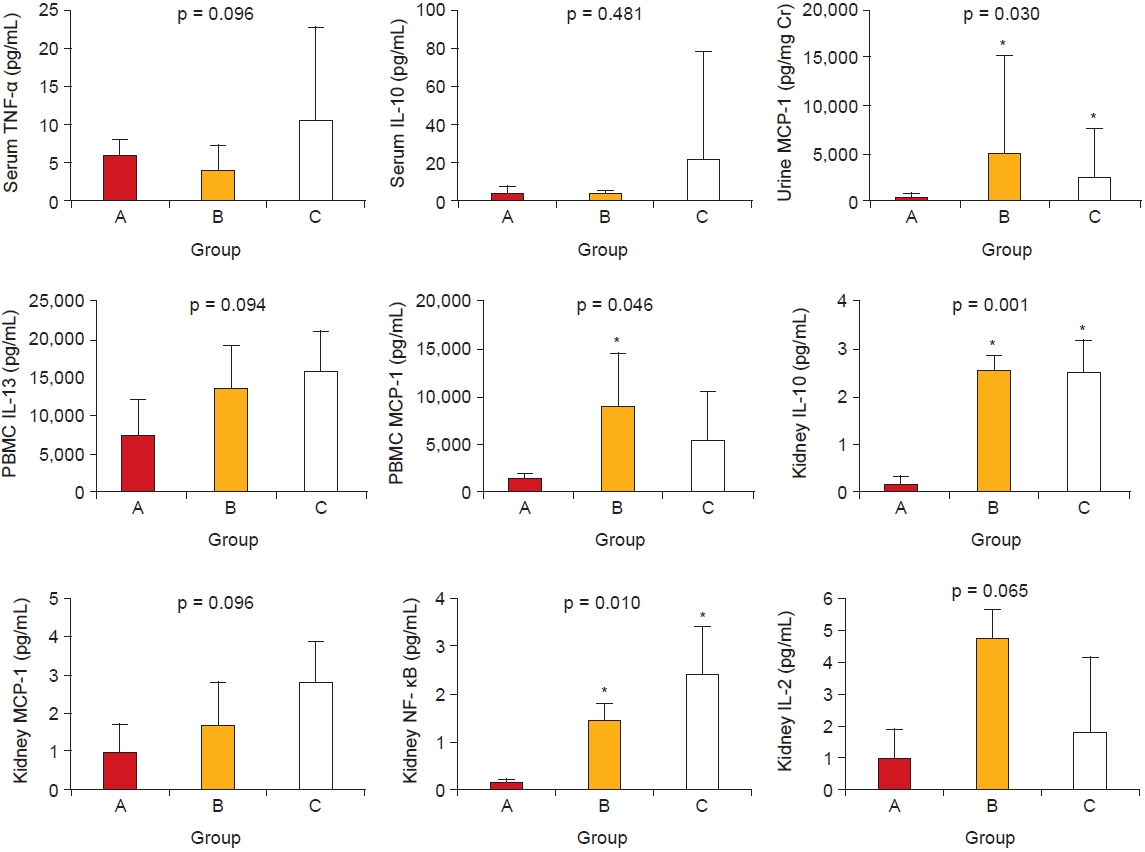
MCP-1 plays an important role in IgAN as well as other glomerulonephritides. Previous studies showed that MCP-1 reflects the disease activity, proteinuria, and severity of chronic histologic changes in IgAN [
16,
17]. In another study, urinary MCP-1 levels independently predicted renal survival [
18]. Although the pathogenetic mechanism of MCP-1 in IgAN is unclear, some reports have shone light on the role of MCP-1 in this disease. Proteinuria and activation of IFN╬│ were suggested as the main stimuli to MCP-1 [
17,
19]. In our study, MCP-1 expression was highly upregulated in several samples from patients with IgAN, especially those with high proteinuria and low eGFR. In addition, the urine MCP-1 level was further correlated with responsiveness to glucocorticoid treatment. These results suggest that MCP-1 might be a valuable marker for determining the disease activity and treatment response in patients with IgAN.
Notably, few studies have shown the relationship between cytokines and disease severity in IgAN. In addition to MCP-1, various proinflammatory (TNF-╬▒, IL-1╬▓), Th1 (IFN╬│, IL-2), and Th2 (IL-4, IL-10) cytokines are related to disease activity in IgAN [
7,
20,
21]. In accordance with these findings, we found that the levels of TNF-╬▒, IL-10, IL-13, and NF-╬║B in various samples were significantly related to disease activity.
Glucocorticoids inhibit various cytokines, but their effect in patients with IgAN is still controversial. Several cytokines, especially IL-6, were found to be suppressed in IgAN after steroid treatment [
22]. However, Kalliakmani et al. [
23] reported that levels of IL-6, a profibrotic cytokine, were unchanged in patients with IgAN after steroid treatment. In our study, levels of urine MCP-1, serum IL-4, and IFN╬│ and IL-2 secreted by PBMCs were significantly correlated with the percentage of proteinuria reduction in patients with IgAN after glucocorticoid treatment. Furthermore, the intrarenal IL-1 mRNA level was correlated with proteinuria reduction, in agreement with the findings of Pathak et al. [
24]. These results highlight the usefulness of cytokine levels assessed in various samples, including PBMCs, in predicting responses to steroid treatment.
Our study has several limitations. First, the small number of patients enrolled in this study does not allow the characterization of disease status based on pathology and the determination of treatment responses. Second, longitudinal data were not enough to determine the relationship between clinical prognosis and cytokine levels. Third, we measured only the intrarenal levels of cytokine mRNA, not that of proteins, which does not reflect the actual expression of cytokines. Regardless of these limitations, the present study offers a comprehensive view of the cytokine profile of patients with IgAN, as it was simultaneously performed in serum, urine, kidney tissue, PBMC, and MC samples.
In conclusion, IFN╬│ was found to be a key cytokine in the specific pathogenetic processes of IgAN. The upregulation of IFN╬│ induced increased urinary MCP-1 production, which in turn correlated with several clinical findings and reduction of proteinuria in patients with IgAN after glucocorticoid treatment. Our data suggest that an integrated analysis of various tissue samples from patients with IgAN would be of utmost importance to further clarify the pathogenetic mechanism underlying this disease.









 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















