| Kidney Res Clin Pract > Volume 41(4); 2022 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202, 2019E320100, 2019E320101, 2019E320102, 2019-ER-7304-00, 2019-ER-7304-01, 2019-ER-7304-02).
Authors’ contributions
Conceptualization: CA, YKO
Data curation: HCP, HR, YCK, JL, YHK, DWC, WKC
Formal analysis: HCP
Funding acquisition: KHO
Investigation: YH, JHY, HR
Methodology: YH, JHY
Supervision: CA, KHO, YKO
Visualization: JHY
Writing–original draft: HCP, YH, JHY, HR, YCK,
Writing–review & editing: JL, YHK, DWC, WKC, CA, KHO, YKO
All authors read and approved the final manuscript.
Acknowledgments
Figure 1.
Study population.

Figure 2.
Correlation between TKVe and TKVs.
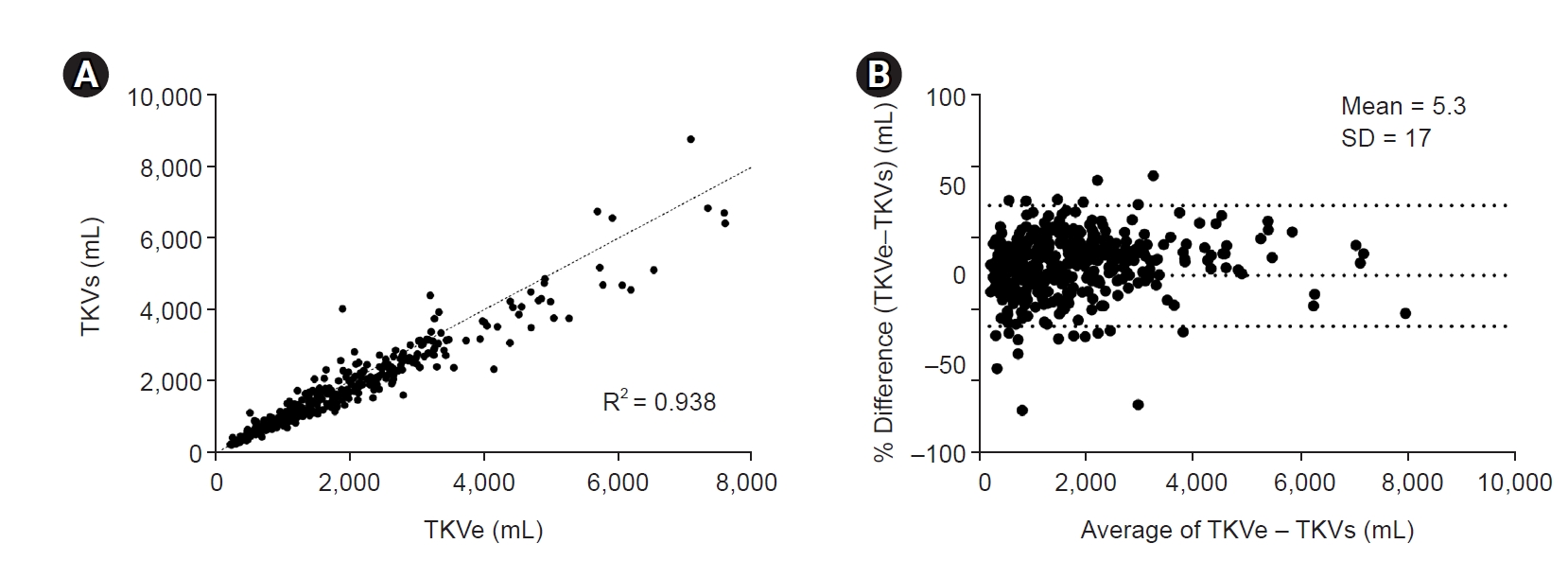
Figure 3.
Difference between eHTKV-α at initial and final points of TKV measurement.
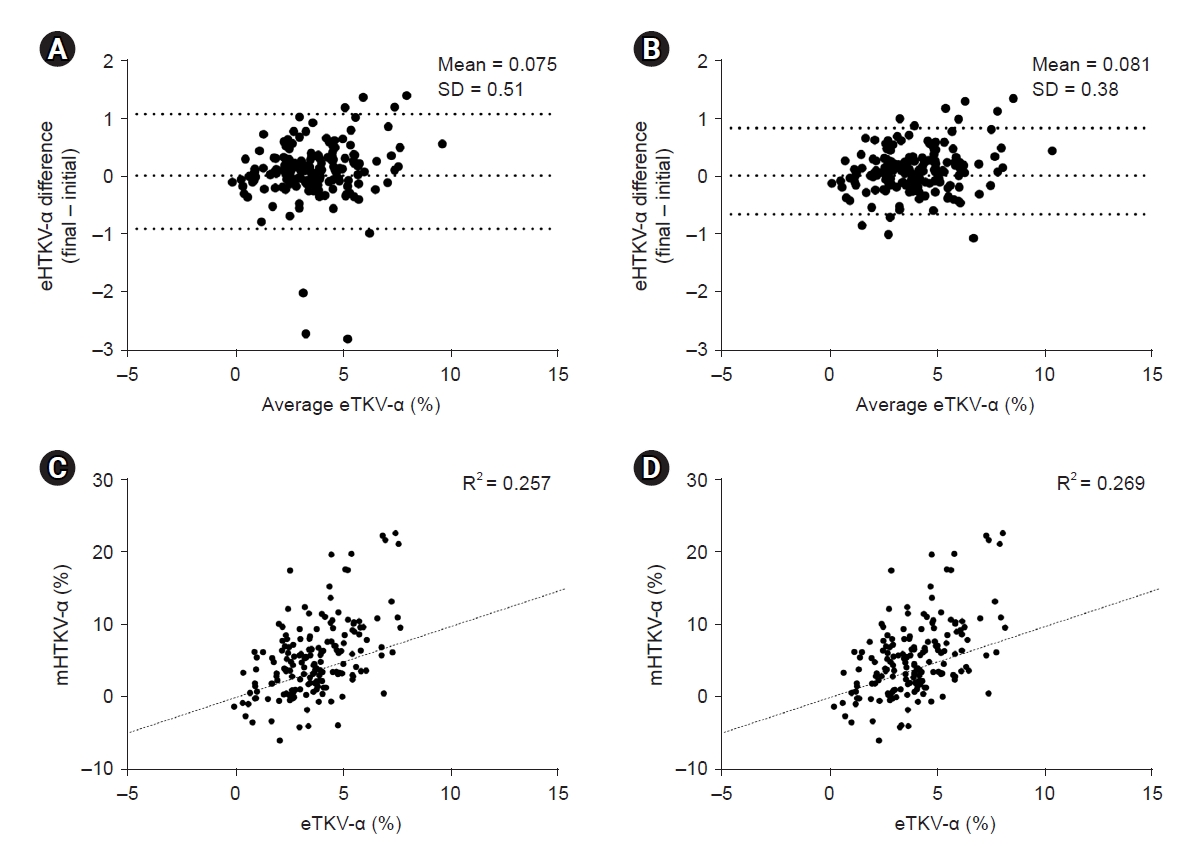
Figure 4.
Renal outcome by the original MIC (A = 0 and K = 150).
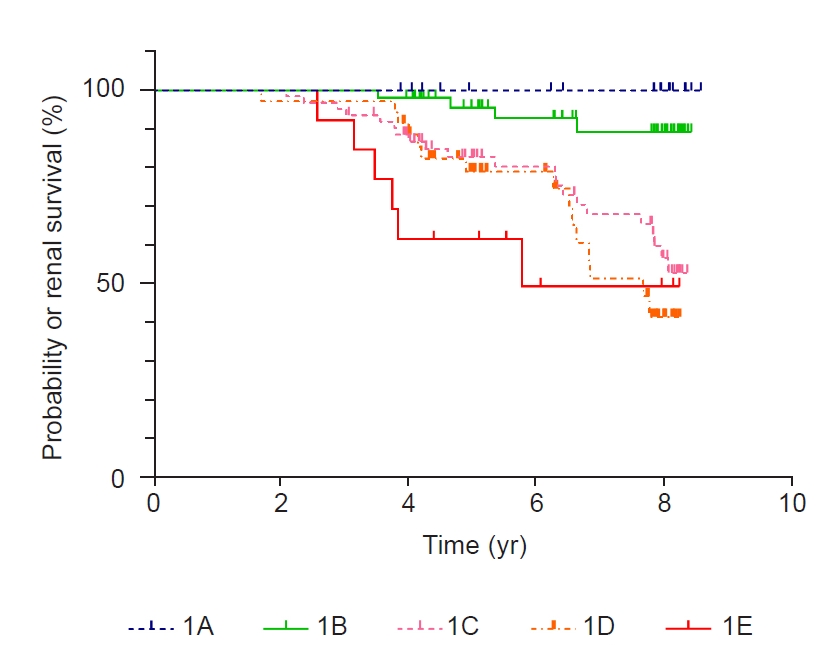
Table 1.
Table 2.
| Variable | HR (95% CI) | p-value |
|---|---|---|
| Age (yr) | 0.97 (0.93–1.02) | 0.12 |
| Male sex (vs. female sex) | 0.78 (0.39–1.55) | 0.47 |
| Body mass index (kg/m2) | 0.95 (0.82–1.09) | 0.44 |
| Systolic BP (mmHg) | 1.00 (0.97–1.03) | 0.98 |
| Serum uric acid (mg/dL) | 1.12 (0.84–1.45) | 0.43 |
| Baseline eGFR (mL/min/1.73 m2) | 0.94 (0.92–0.96) | <0.001 |
| Macroalbuminuria (vs. normoalbuminuria or microalbuminuria) | 3.53 (1.66–7.49) | 0.001 |
| PKD1 genotype (vs. PKD2) | 2.45 (0.71–8.44) | 0.16 |
| Rapid progressora (vs. slow progressor) | 4.09 (1.23–13.54) | 0.02 |
Table 3.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Hayne Cho Park

https://orcid.org/0000-0002-1128-3750Yeji Hong

https://orcid.org/0000-0001-7182-9789Jeong-Heum Yeon

https://orcid.org/0000-0002-8486-9832Hyunjin Ryu

https://orcid.org/0000-0003-2148-4465Yong-Chul Kim

https://orcid.org/0000-0003-3215-8681Joongyub Lee

https://orcid.org/0000-0003-2784-3772Yeong Hoon Kim

https://orcid.org/0000-0002-4101-9993Dong-Wan Chae

https://orcid.org/0000-0001-9401-892XWooKyung Chung

https://orcid.org/0000-0001-7657-130XCurie Ahn

https://orcid.org/0000-0001-7033-1102Kook-Hwan Oh

https://orcid.org/0000-0001-9525-2179Yun Kyu Oh

https://orcid.org/0000-0001-8632-5743 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















