| Kidney Res Clin Pract > Volume 42(2); 2023 > Article |
|
Abstract
Background
Methods
Results
Supplementary Materials
Notes
Conflicts of interest
Tae-Hyun Yoo is the Editor-in-Chief of Kidney Research and Clinical Practice and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
Funding
This work was supported by the Research Program funded by the Korea Disease Control and Prevention Agency (2011E3300300, 2012E3301100, 2013E3301600, 2013E3301601, 2013E3301602, 2016E3300200, 2016E3300201, 2016E3300202,2019E320100, 2019E320101, 2019E320102, 2022-11-007), by the National Research Foundation of Korea (NRF) funded by the Korea government (MSIT) (NRF-2019R1A2C2086276), and a grant (BCRI22042 and BCRI22079) of Chonnam National University Hospital Biomedical Research Institute.
Figure 1.
Flow diagram of the study participants.
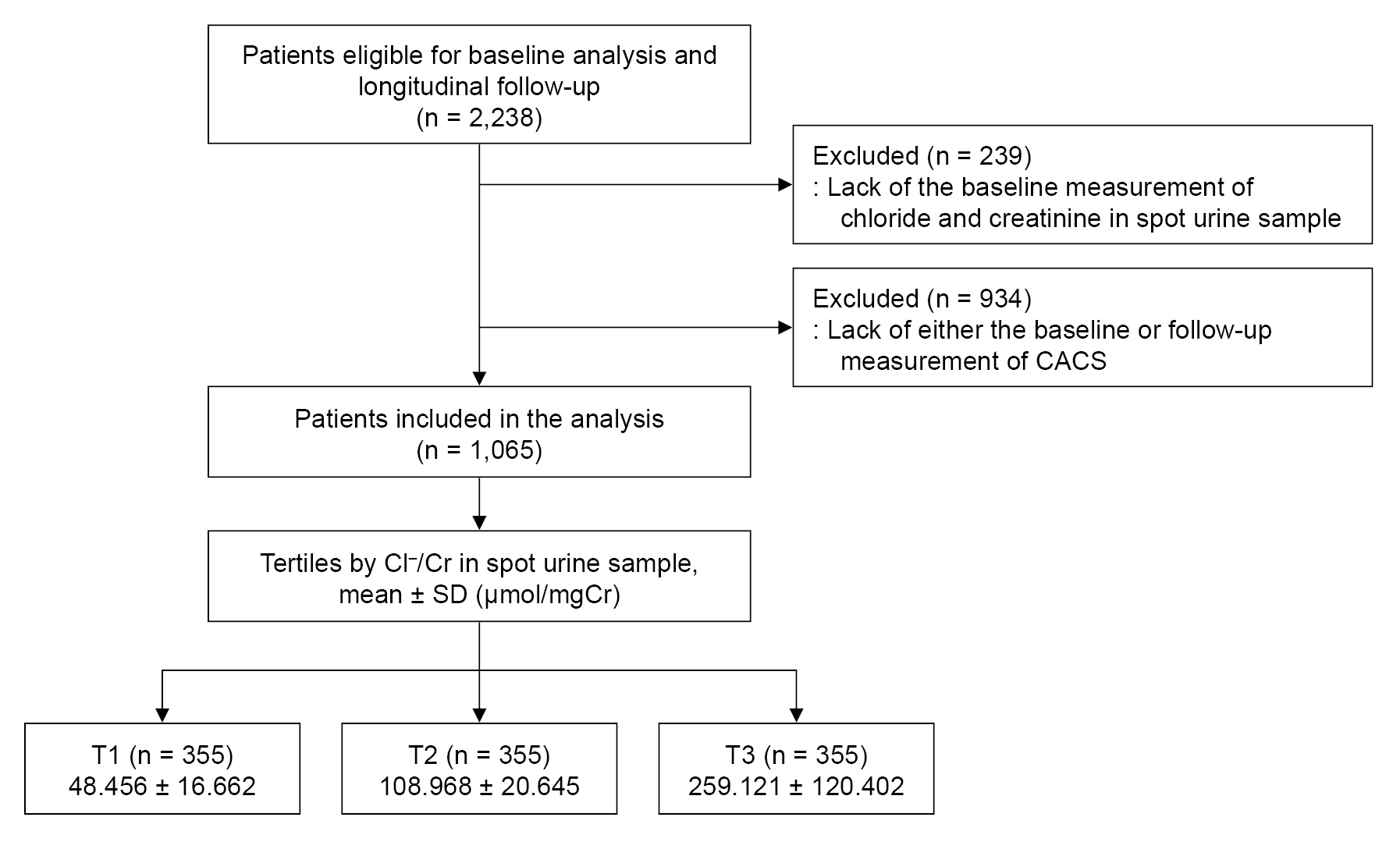
Figure 2.
Restricted cubic spline of spot urine Cl–/Cr on the risk of CAC progression.
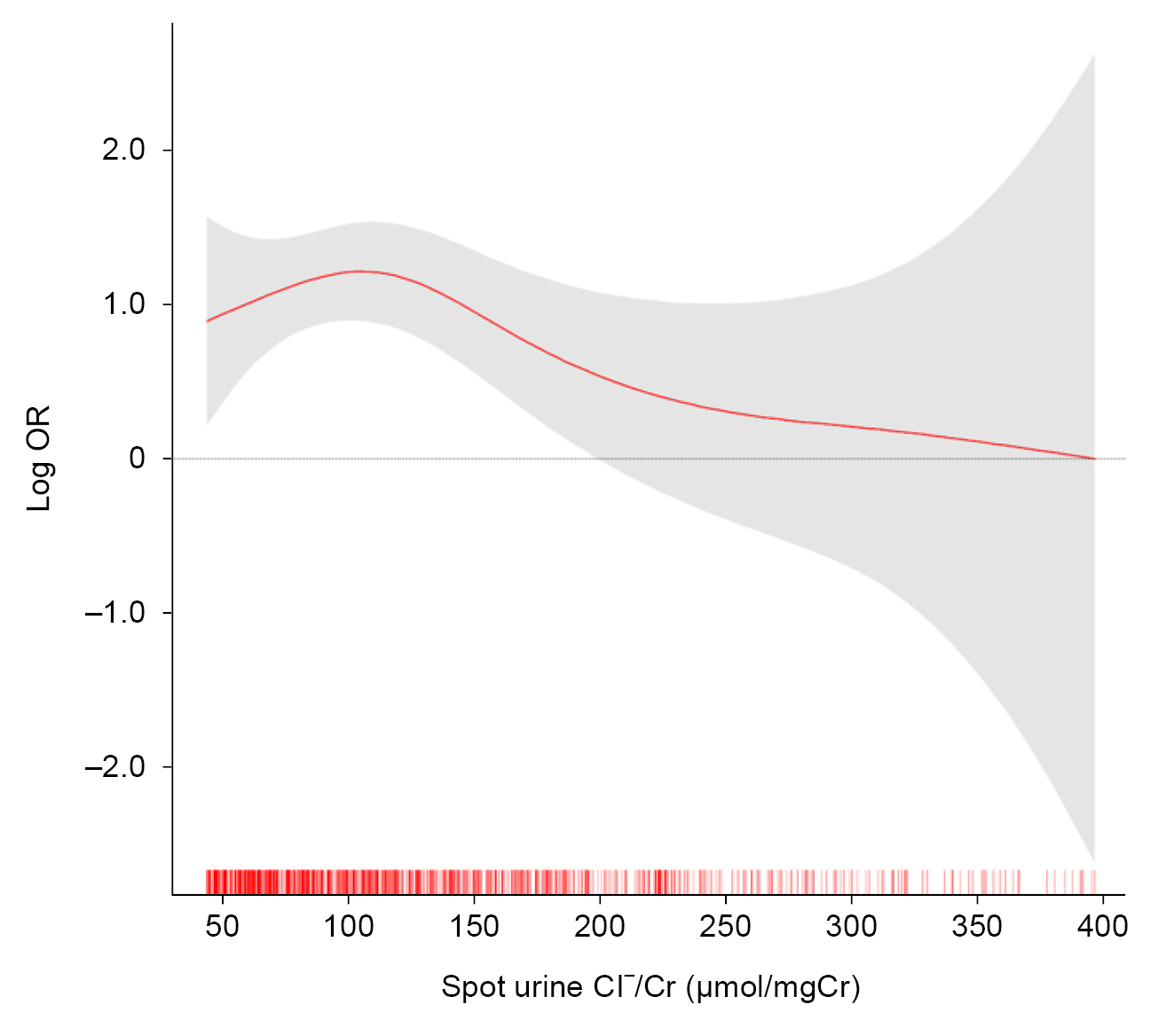
Table 1.
Data are expressed as number (%), mean ± standard deviation, or median (interquartile range).
ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; AU, Agatston unit; CACS, coronary artery calcium score; CKD, chronic kidney disease; Cl–/Cr, chloride-to-creatinine ratio; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; HTN, hypertension; K+/Cr, potassium-to-creatinine ratio; LDC-C, low-density lipoprotein cholesterol; Na+/Cr, sodium-to-creatinine ratio; PKD, polycystic kidney disease; SBP, systolic blood pressure; T1, 1st tertile; T2, 2nd tertile; T3, 3rd tertile; TC, total cholesterol; TG, triglyceride; TID, tubulointerstitial disease.
Table 2.
Model 1: unadjusted model. Model 2: model 1 + adjusted for age, sex, Charlson comorbidity index, primary renal disease, current smoking status, medication (ACEi/ARBs, diuretics, number of anti-HTN drugs, statins), BMI, and SBP. Model 3: model 2 + adjusted for hemoglobin, albumin, fasting glucose, HDL-C, TG, 25(OH) vitamin D, hsCRP, eGFR, and spot urine ACR. Model 4: model 3 + adjusted for CACS at the baseline.
ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; CAC, coronary artery calcification; CACS, coronary artery calcium score; CI, confidence interval; Cl–/Cr, chloride-to-creatinine ratio; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; HTN, hypertension; OR, odds ratio; SBP, systolic blood pressure; T1, 1st tertile; T2, 2nd tertile; T3, 3rd tertile; TG, triglyceride.
Table 3.
Model 1: unadjusted model. Model 2: model 1 + adjusted for age, sex, Charlson comorbidity index, primary renal disease, current smoking status, medication (ACEi/ARBs, diuretics, number of anti-HTN drugs, statins), BMI, and SBP. Model 3: model 2 + adjusted for hemoglobin, albumin, fasting glucose, HDL-C, TG, 25(OH) vitamin D, hsCRP, eGFR, and spot urine ACR. Model 4: model 3 + adjusted for CACS at the baseline.
ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; CAC, coronary artery calcification; CACS, coronary artery calcium score; CI, confidence interval; Cl–/Cr, chloride-to-creatinine ratio; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; HTN, hypertension; OR, odds ratio; SBP, systolic blood pressure; T1, 1st tertile; T2, 2nd tertile; T3, 3rd tertile; TG, triglyceride.
Table 4.
The model was adjusted for age, sex, Charlson comorbidity index, primary renal disease, current smoking status, medication (ACEi/ARBs, diuretics, number of anti-HTN drugs, statins), BMI, SBP, hemoglobin, albumin, fasting glucose, HDL-C, TG, 25(OH) vitamin D, hsCRP, eGFR, spot urine ACR, and CACS at the baseline.
ACEi, angiotensin-converting enzyme inhibitor; ACR, albumin-to-creatinine ratio; ARB, angiotensin receptor blocker; BMI, body mass index; CAC, coronary artery calcification; CACS, coronary artery calcium score; CI, confidence interval; Cl–/Cr, chloride-to-creatinine ratio; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; hsCRP, high-sensitivity C-reactive protein; HTN, hypertension; OR, odds ratio; SBP, systolic blood pressure; T1, 1st tertile; T2, 2nd tertile; T3, 3rd tertile; TG, triglyceride.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Sang Heon Suh

https://orcid.org/0000-0003-3076-3466Tae Ryom Oh

https://orcid.org/0000-0002-3713-0939Hong Sang Choi

https://orcid.org/0000-0001-8191-4071Chang Seong Kim

https://orcid.org/0000-0001-8753-7641Eun Hui Bae

https://orcid.org/0000-0003-1727-2822Seong Kwon Ma

https://orcid.org/0000-0002-5758-8189Kook-Hwan Oh

https://orcid.org/0000-0001-9525-2179Tae-Hyun Yoo

https://orcid.org/0000-0002-9183-4507Dong-Wan Chae

https://orcid.org/0000-0001-9401-892XSoo Wan Kim

https://orcid.org/0000-0002-3540-9004 - Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print















