| Kidney Res Clin Pract > Volume 41(5); 2022 > Article |
|
Abstract
Background
Methods
Results
Notes
Funding
This research was supported by the grant from the Seoul National University Bundang Hospital Research Fund (No. 02-2020-026).
Authors’ contributions
Conceptualization, Formal analysis, Funding acquisition: JCJ
Data curation: HES, JYR, KL, YIC, MSK, JCJ
Methodology: HES, SA, SSH
Project administration: HK, JCJ, CA
Supervision: IP, GTS, HK, SK, HJC, KYN, DWC
Resources, Software: SK
Writing–original draft: HES, JCJ, SSH
Writing–review & editing: JYR, KL, YIC, MSK, IP, GTS, HK, CA, SK, HJC, KYN, DWC, SA
All authors read and approved the final manuscript.
Acknowledgments
Figure 2.
Restricted cubic spline curve of odds according to change of the percentage of SMM to WT (A) and the change of HGS (B).
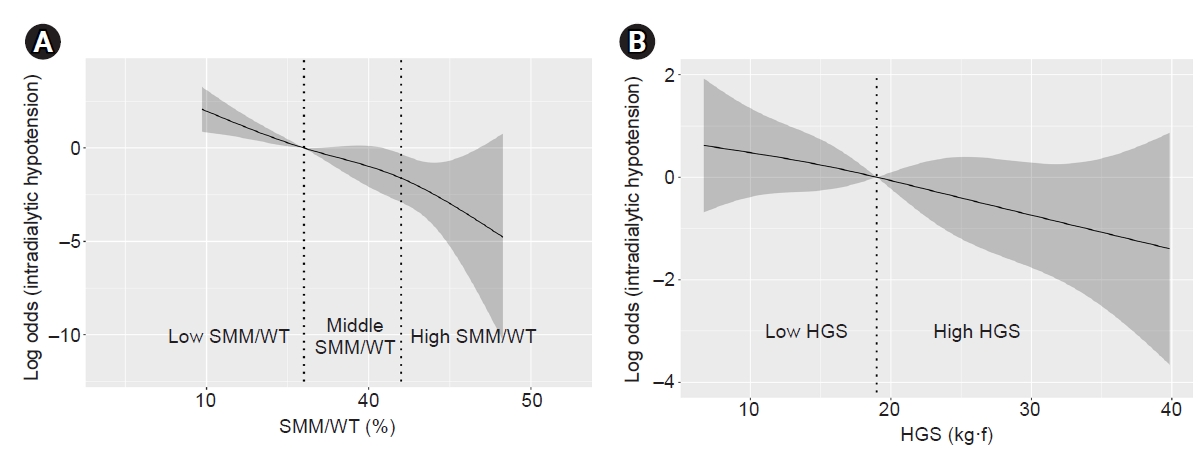
Table 1.
| Characteristic | Total (n = 177) |
Intradialytic hypotension |
p-value | |
|---|---|---|---|---|
| Yes (n = 39) | No (n = 138) | |||
| Age (yr) | 59.5 ± 14.5 | 62.1 ± 11.2 | 58.8 ± 15.3 | 0.22 |
| Male sex | 87 (49.2) | 13 (33.3) | 74 (53.6) | 0.03 |
| Weight (kg) | 59.3 ± 11.0 | 60.2 ± 12.0 | 59.1 ± 10.8 | 0.58 |
| Height (cm) | 161.8 ± 8.6 | 159.8 ± 8.6 | 162.4 ± 8.5 | <0.001 |
| Body mass index (kg/m2) | 22.5 ± 3.3 | 23.5 ± 4.2 | 22.2 ± 2.9 | 0.03 |
| Duration of hemodialysis (yr) | 5.5 ± 4.3 | 5.1 ± 3.1 | 5.6 ± 4.6 | 0.50 |
| Etiology of ESRD | <0.001 | |||
| Diabetes mellitus | 66 (37.3) | 27 (69.2) | 39 (28.3) | |
| Hypertension | 28 (15.8) | 2 (5.1) | 26 (18.8) | |
| Glomerulonephritis | 35 (19.8) | 3 (7.7) | 32 (23.2) | |
| Others | 24 (13.6) | 2 (5.1) | 22 (15.9) | |
| Unknown | 24 (13.6) | 5 (12.8) | 19 (13.8) | |
| Hypertension | 150 (84.7) | 35 (89.7) | 115 (83.3) | 0.33 |
| Diabetes mellitus | 81 (45.8) | 30 (76.9) | 51 (37.0) | <0.001 |
| Ischemic heart disease | 35 (19.8) | 14 (35.9) | 21 (15.2) | 0.004 |
| Chronic heart failure | 15 (8.5) | 7 (17.9) | 8 (5.8) | 0.02 |
| Stroke | 19 (10.7) | 8 (20.5) | 11 (8.0) | 0.03 |
| Peripheral arterial occlusive disease | 10 (5.6) | 6 (15.4) | 4 (2.9) | 0.003 |
| NT-proBNP (pg/mL) | 9,359.3 ± 9,705.2 | 10,249.2 ± 10,136.7 | 9,107.9 ± 9,602.9 | 0.52 |
| Cardiac index (%) | 51.7 ± 6.9 | 51.9 ± 5.0 | 51.7 ± 7.4 | 0.85 |
| Hemoglobin (g/dL) | 10.4 ± 0.9 | 10.6 ± 1.0 | 10.3 ± 0.9 | 0.20 |
| Blood urine nitrogen (mg/dL) | 61.0 ± 15.8 | 63.1 ± 15.9 | 60.4 ± 15.7 | 0.33 |
| Creatinine (mg/dL) | 9.9 ± 3.0 | 8.8 ± 2.6 | 10.2 ± 3.1 | 0.01 |
| Calcium (mg/dL) | 9.0 ± 0.6 | 8.9 ± 0.6 | 9.0±0.7 | 0.53 |
| Sodium (mEq/L) | 137.5 ± 3.3 | 136.8 ± 3.3 | 137.7 ± 3.2 | 0.15 |
| Phosphate (mg/dL) | 5.2 ± 1.5 | 5.4 ± 1.6 | 5.1 ± 1.4 | 0.33 |
| Albumin (g/dL) | 3.9 ± 0.4 | 3.9 ± 0.4 | 3.9 ± 0.4 | 0.86 |
| Total cholesterol (mg/dL) | 148.4 ± 33.7 | 150.6 ± 39.8 | 147.7 ± 31.9 | 0.64 |
| Low-density lipoprotein (mg/dL) | 86.4 ± 72.6 | 109.4 ± 136.8 | 78.4 ± 25.1 | 0.08 |
| Iron (ng/mL) | 65.8 ± 31.0 | 57.5 ± 30.4 | 68.1 ± 30.9 | 0.06 |
| TIBC (μg/dL) | 229.3 ± 46.9 | 233.6 ± 55.3 | 228.1.6 ± 44.3 | 0.52 |
| Ferritin (ng/mL) | 245.3 ± 395.3 | 248.0 ± 198.1 | 244.5 ± 435.8 | 0.96 |
| Potassium (mEq/L) | 5.4 ± 1.0 | 5.7 ± 1.1 | 5.3 ± 1.0 | 0.03 |
| Parathyroid hormone (pg/mL) | 254.5 ± 206.0 | 199.2 ± 157.4 | 270.1 ± 215.7 | 0.06 |
| Ultrafiltration per weight (%) | 4.5 ± 1.7 | 5.1 ± 1.9 | 4.4 ± 1.6 | 0.02 |
| spKt/V | 1.6 ± 0.3 | 1.6 ± 0.4 | 1.6 ± 0.3 | 0.34 |
| PG-SGA score | 3.7 ± 4.4 | 3.3 ± 3.9 | 3.8 ± 4.5 | 0.56 |
| Tilburg frailty score (score) | 3.5 ± 2.5 | 4.1 ± 2.5 | 3.3 ± 2.5 | 0.09 |
| Triceps skinfold thickness (mm) | 17.8 ± 7.9 | 18.7 ± 8.1 | 17.6 ± 7.8 | 0.43 |
| Mid-arm muscle circumference (cm) | 26.3 ± 3.2 | 26.6 ± 3.8 | 26.2 ± 3.1 | 0.53 |
| Handgrip strength (kg·f) | 21.1 ± 10.3 | 16.5 ± 7.9 | 22.4 ± 10.6 | 0.001 |
| Percentage of SMM to WT (%) | 38.9 ± 5.8 | 34.3 ± 4.9 | 40.2 ± 5.3 | <0.001 |
| SMM to squared height (kg/m2) | 8.7 ± 1.2 | 8.9 ± 1.2 | 7.9 ± 1.0 | <0.001 |
| Possible sarcopeniaa | 113 (63.8) | 33 (84.6) | 80 (58.0) | 0.003 |
| Sarcopeniab | 4 (2.3) | 3 (7.7) | 1 (0.7) | 0.01 |
| Extracellular water to TBW (%) | 39.4 ± 1.5 | 40.1 ± 1.3 | 39.2 ± 1.5 | 0.001 |
| Intracellular water to TBW (%) | 60.6 ± 1.5 | 59.8 ± 1.3 | 60.8 ± 1.5 | <0.001 |
| Phase angle at 50 Hz (˚) | 4.9 ± 1.2 | 4.4 ± 0.9 | 5.0 ± 1.2 | 0.002 |
| Medication prescription | ||||
| Antihypertensive | 132 (74.6) | 24 (61.5) | 108 (78.3) | 0.03 |
| No. of medicationsc | 0.23 | |||
| 1–2 | 91 (51.4) | 19 (48.7) | 72 (52.2) | |
| ≥3 | 41 (23.2) | 5 (12.8) | 36 (26.1) | |
| Beta blocker | 110 (62.1) | 21 (53.8) | 89 (64.5) | 0.23 |
| ACE inhibitor | 37 (20.9) | 6 (15.4) | 31 (22.5) | 0.34 |
| ARB | 105 (59.3) | 16 (41.0) | 89 (64.5) | 0.008 |
| Diuretics | 37 (20.9) | 5 (12.8) | 32 (23.2) | 0.16 |
| Calcium-channel blocker | 35 (19.8) | 5 (12.8) | 30 (21.7) | 0.22 |
| Alpha-blocker | 42 (23.7) | 5 (12.8) | 37 (26.8) | 0.07 |
| Vasodilator | 37 (20.9) | 6 (15.4) | 31 (22.5) | 0.34 |
| Total prescribed medicationsd | 2.4 ± 2.7 | 1.7 ± 2.2 | 2.6 ± 2.8 | 0.09 |
Data are presented as mean ± standard deviation, number (%), or number only.
Cutoff values to classify sarcopenia followed the criteria of the Asian Working Group.
ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; ESRD, end-stage renal disease; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; SMM, skeletal muscle mass; spKt/V, single-pool ratio of urea clearance multiplied by dialysis time to volume; PG-SGA, patient-generated subjective global assessment; TBW, total body water; TIBC, total iron binding capacity; WT, dry body weight.
a Possible sarcopenia was defined as low handgrip strength (<28 kg•f in males and <18 kg•f in females).
Table 2.
| Variable |
OR (95% CI) of IDH among groups divided by SMM/WT |
||
|---|---|---|---|
| Low (reference) | Middle | High | |
| Unadjusted | Reference | 0.37 (0.16–0.85)a | 0.08 (0.02–0.28)b |
| Multivariable regressionc | Reference | 0.30 (0.10–0.88)a | 0.06 (0.01–0.29)b |
| IPTWc | Reference | 0.83 (0.69–1.00) | 0.71 (0.59–0.85)b |
Patients were grouped by rank numbering in order of the percentage of SMM (kg), measured by bioimpedance analysis, to WT (kg), as tertile groups of low, middle, and high percentage of SMM to WT. OR to IDH were analyzed using logistic models before and after weighting.
CI, confidence interval; IDH, intradialytic hypotension; IPTW, inverse probability of treatment weighting; OR, odds ratio; SMM, skeletal muscle mass; SMM/WT, percentage of SMM to WT; WT, dry body weight.
Table 3.
| Variable | OR (95% CI) |
|---|---|
| SMM/WT (%) plus HGS (kg·f) (vs. SMM/WT, ≥36.0 and HGS, ≥19.0) | |
| Either SMM/WT, <36.0 or HGS, <19.0 | 4.44 (1.29–15.33) |
| SMM/WT, <36.0 and HGS, <19.0 | 17.11 (3.47–84.27) |
| Age | 1.00 (0.96–1.04) |
| Male sex (vs. female) | 1.47 (0.45–4.80) |
| DM (vs. non-DM) | 5.68 (2.20–14.65) |
| Cardiovascular comorbiditiesa (yes vs. no) | 2.26 (0.90–5.69) |
| Cardiac index | 0.95 (0.88–1.03) |
| Ultrafiltration per weight | 1.60 (1.18–2.16) |
To classify SMM/WT and HGS, we used the highest value in the low-SMM/WT tertile group (36.0%), and the median value of HGS (19.0 kg•f) showed an OR of 17.11 to IDH compared to that in patients with SMM/WT of ≥36.0% and HGS of ≥19.0 kg•f.
CI, confidence interval; DM, diabetes mellitus; HGS, handgrip strength; IDH, intradialytic hypotension; OR, odds ratio; SMM, skeletal muscle mass; SMM/WT, percentage of SMM to WT; WT, dry body weight.
Table 4.
| Model including | ROC area (95% CI) |
|---|---|
| Clinical parameters + SMM/WT + HGS | 0.88 (0.83–0.94)b |
| Clinical parameters + SMM/WT | 0.88 (0.83–0.93)b |
| Clinical parameters + SMM to squared height | 0.85 (0.78–0.91) |
| Clinical parameters + ECW to TBW | 0.81 (0.74–0.88) |
| Clinical parameters + ICW to TBW | 0.81 (0.74–0.89) |
| Clinical parameters + PA | 0.81 (0.74–0.89) |
| Clinical parameters + HGS | 0.81 (0.74–0.88) |
| Clinical parameters only | 0.81 (0.74–0.88) |
Parameters measured by bioimpedance analysis were included as continuous variables in each multivariable logistic model. Clinical parameters were age, sex, cardiovascular comorbidities (chronic heart failure or ischemic heart disease), diabetes mellitus, cardiac index, and percentage of ultrafiltration to body weight. The AUC was statistically higher in models including the percentage of SMM (kg) to WT (kg) compared with those of other models.
AUC, area under the ROC curve; CI, confidence interval; ECW, extracellular water; HGS, handgrip strength; ICW, intracellular water; PA, phase angle; ROC, receiver operating characteristic; SMM, skeletal muscle mass; SMM/WT, percentage of SMM to WT; TBW, total body water; WT, dry body weight.
References
- TOOLS
-
METRICS

- ORCID iDs
-
Hyung Eun Son

https://orcid.org/0000-0002-8719-3823Ji Young Ryu

https://orcid.org/0000-0003-4134-1007Kyunghoon Lee

https://orcid.org/0000-0002-3154-0347Young Il Choi

https://orcid.org/0000-0002-1150-9553Myeong Sung Kim

https://orcid.org/0000-0001-9870-9605Inwhee Park

https://orcid.org/0000-0002-9912-5393Gyu Tae Shin

https://orcid.org/0000-0002-1343-5332Heungsoo Kim

https://orcid.org/0000-0002-9380-7457Curie Ahn

https://orcid.org/0000-0001-7033-1102Sejoong Kim

https://orcid.org/0000-0002-7238-9962Ho Jun Chin

https://orcid.org/0000-0002-3710-0190Ki Young Na

https://orcid.org/0000-0002-8872-8236Dong-Wan Chae

https://orcid.org/0000-0001-9401-892XSoyeon Ahn

https://orcid.org/0000-0003-3440-2027Seung Sik Hwang

https://orcid.org/0000-0002-1558-7831Jong Cheol Jeong

https://orcid.org/0000-0003-0301-7644 - Related articles
-
Recurrent Diabetic Muscle Infarction in A Patient on Maintenance Hemodialysis2007 July;26(4)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















