| Kidney Res Clin Pract > Volume 41(5); 2022 > Article |
|
Abstract
Background
Methods
Results
Notes
Conflicts of interest
Tae-Hyun Yoo is the Editor-in-Chief of Kidney Research and Clinical Practice and was not involved in the review process of this article. All authors have no other conflicts of interest to declare.
Funding
This study was supported by a cooperative research fund from the Korean Nephrology Research Foundation (2021). The sponsor had no role in the study design, data collection, or analysis.
AuthorsŌĆÖ contributions
Conceptualization: YSJ, HWK, HL, KCM, SHH
Data curation: YSJ, HWK, KCM, HJC, SHH
Formal analysis: YSJ, HWK, CHB, SHH
Funding acquisition: HL
Investigation: YSJ, JTP, SHH
Methodology: YSJ, CHB, JTP, HL, SHH
Project administration: HL, HJC
Visualization: YSJ, HWK, HL, SHH
Validation: YSJ, HL, SHH
Resources: HL, SHH
Supervision: BJL, THY, SWK, SHH
WritingŌĆōoriginal draft: YSJ, HWK, JTP, HL, BJL, THY, KCM, HJC, SWK, SHH
WritingŌĆōreview & editing: All authors
All authors read and approved the final manuscript.
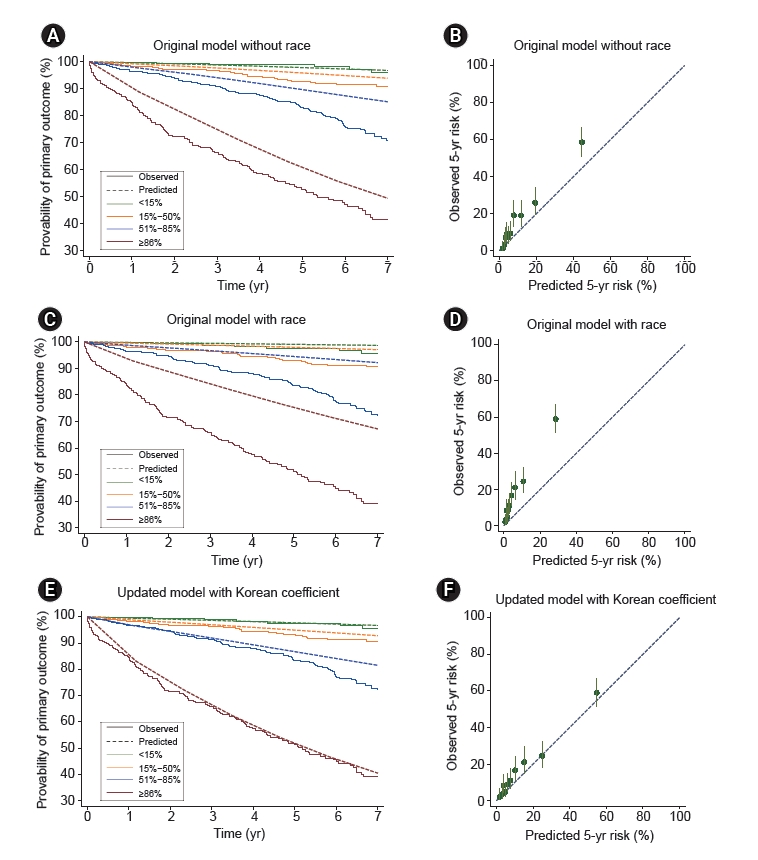
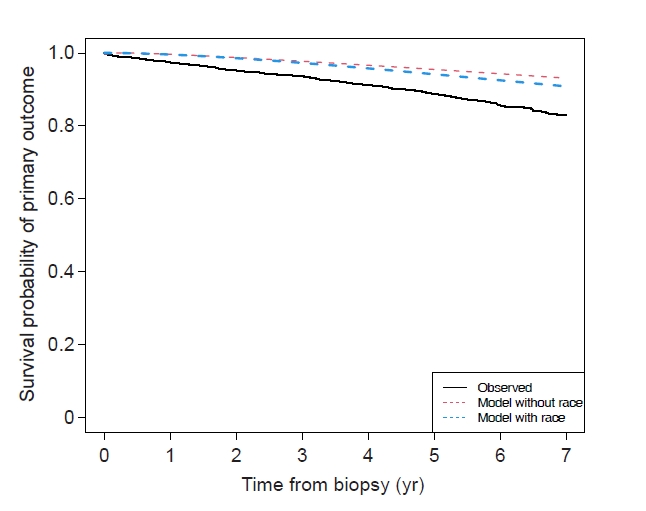
Figure┬Ā1.
Calibration plots.
Table┬Ā1.
| Characteristic | Korean cohort |
Original cohorta) |
|
|---|---|---|---|
| Derivation cohort | Validation cohort | ||
| No. of patients | 2,064 | 2,781 | 1,146 |
| ŌĆāAsan Hospital | 298 (14.4) | ||
| ŌĆāSeverance Hospital | 678 (32.8) | ||
| ŌĆāSeoul National University Hospital | 623 (30.2) | ||
| ŌĆāSeoul National University Bundang Hospital | 465 (22.5) | ||
| Year of biopsy | 2014 (2011ŌĆō2016) | 2006 (2004ŌĆō2008) | 1998 (1993ŌĆō2003) |
| Follow-up (yr) | 3.8 (1.8ŌĆō6.6) | 4.8 (3.0ŌĆō7.6) | 5.8 (3.4ŌĆō8.5) |
| Age (yr) | 41.3 ┬▒ 14.2 | ||
| Male sex | 925 (44.8) | 1,608 (57.8) | 565 (49.3) |
| Creatinine level at biopsy (mg/dL) | 0.93 (0.73ŌĆō1.24) | 1.04 (0.80ŌĆō1.40) | 0.95 (0.75ŌĆō1.26) |
| eGFR at biopsy (mL/min/1.73 m2) | 87.8 (61.6ŌĆō111.5) | 83.0 (56.7ŌĆō108.0) | 89.7 (65.3ŌĆō112.7) |
| ŌĆā<30 | 94 (4.6) | 142 (5.1) | 37 (3.2) |
| ŌĆā30ŌĆō60 | 401 (19.4) | 657 (23.6) | 191 (16.7) |
| ŌĆā60ŌĆō90 | 584 (28.3) | 800 (28) | 350 (30.5) |
| ŌĆā>90 | 985 (47.7) | 1,182 (42.5) | 568 (49.6) |
| Mean arterial blood pressure (mmHg) | 92 (83ŌĆō100) | 97 (89ŌĆō106) | 93 (85ŌĆō103) |
| Proteinuria at biopsy (g/day) | 1.0 (0.5ŌĆō1.9) | 1.2 (0.7ŌĆō2.2) | 1.3 (0.6ŌĆō2.4) |
| ŌĆā<0.5 | 530 (25.7) | 383 (13.9) | 221 (19.3) |
| ŌĆā0.5ŌĆō1 | 488 (23.6) | 772 (28.1) | 209 (18.2) |
| ŌĆā1ŌĆō2 | 570 (27.6) | 817 (29.7) | 352 (30.7) |
| ŌĆā2ŌĆō3 | 215 (10.4) | 360 (13.1) | 145 (12.7) |
| ŌĆā>3 | 261 (12.6) | 415 (15.1) | 215 (18.8) |
| Body mass index (m2/kg) | 23.2 (21.0ŌĆō25.8) | 23.8 (21.3ŌĆō26.6) | 22.8 (20.2ŌĆō25.3) |
| Pathology (MEST-C score) | |||
| ŌĆāM | 755 (36.6) | 1,054 (37.9) | 481 (42.0) |
| ŌĆāE | 471 (22.8) | 478 (17.2) | 476 (41.5) |
| ŌĆāS | 1,350 (65.4) | 2,137 (77.0) | 912 (79.6) |
| ŌĆāT1 | 452 (21.9) | 686 (24.7) | 207 (18.1) |
| ŌĆāT2 | 91 (4.4) | 128 (4.6) | 122 (10.6) |
| ŌĆāC | 320 (19.8) | 953 (34.3) | 642 (56.1) |
| RASB use at biopsy | 644 (31.2) | 862 (32.4) | 320 (30.0) |
| Immunosuppression use | |||
| ŌĆāAt biopsy | 196 (9.5) | 252 (9.1) | 81 (7.1) |
| ŌĆāAfter biopsy | 366 (17.7) | 1,209 (43.5) | 359 (31.3) |
| ŌĆāTime from biopsy to onset of immunosuppression (mo) | 1.7 (0.0ŌĆō19.6) | 1.6 (0.0ŌĆō5.1) | 1.2 (0.0ŌĆō11.5) |
| Primary outcome | |||
| ŌĆā50% decline in eGFR | 331 (16.0) | 420 (15.1) | 210 (18.3) |
| ŌĆāEnd-stage kidney disease | 200 (9.7) | 372 (13.4) | 155 (13.5) |
| ŌĆāPrimary outcome development | 353 (17.1) | 492 (17.7) | 213 (18.6) |
Data are expressed as number only, number (%), median (interquartile range), or mean ┬▒ standard deviation.
The primary outcome was the first occurrence of either a permanent 50% decline in eGFR from the baseline level at biopsy or ESKD. ESKD was defined as the initiation of renal replacement therapy, including dialysis, renal transplantation, or eGFR of <15 mL/min/1.73 m2.
eGFR, estimated glomerular filtration rate; MEST-C, mesangial (M) and endocapillary (E) hypercellularity, segmental sclerosis (S), interstitial fibrosis/tubular atrophy (T), and crescents (C); RASB, renin-angiotensin system blocker.
a Barbour et al. [18] (JAMA Intern Med 2019;179:942-952).
Table┬Ā2.
Table┬Ā3.
| Risk subgroup | Predicted 5-year riska (%) | Observed events in 5 yearsb | Rate of eGFR declinec (mL/min/1.73 m2/yr) | p-value |
|---|---|---|---|---|
| Model without race | <0.001 | |||
| ŌĆāLow risk | 2.1 (0.6ŌĆō2.7) | 3 (1.0) | ŌĆō1.04 (ŌĆō1.56 to ŌĆō0.52) | |
| ŌĆāIntermediate risk | 3.9 (2.7ŌĆō5.4) | 37 (5.1) | ŌĆō1.61 (ŌĆō1.94 to ŌĆō1.28) | |
| ŌĆāHigh risk | 9.6 (5.4ŌĆō18.8) | 85 (11.8) | ŌĆō1.91 (ŌĆō2.24 to ŌĆō1.58) | |
| ŌĆāHighest risk | 37.1 (18.8ŌĆō90.6) | 121 (39.2) | ŌĆō3.24 (ŌĆō3.77 to ŌĆō2.70) | |
| Model with race | <0.001 | |||
| ŌĆāLow risk | 0.8 (0.2ŌĆō1.2) | 5 (1.6) | ŌĆō1.13 (ŌĆō1.64 to ŌĆō0.62) | |
| ŌĆāIntermediate risk | 1.9 (1.2ŌĆō2.7) | 35 (4.8) | ŌĆō1.65 (ŌĆō1.98 to ŌĆō1.31) | |
| ŌĆāHigh risk | 5.1 (2.7ŌĆō10.3) | 82 (11.3) | ŌĆō1.81 (ŌĆō2.14 to ŌĆō1.48) | |
| ŌĆāHighest risk | 23.3 (10.3ŌĆō76.5) | 124 (40.1) | ŌĆō3.38 (ŌĆō3.92 to ŌĆō2.84) |
References
- TOOLS
-
METRICS

- ORCID iDs
-
Young Su Joo

https://orcid.org/0000-0002-7890-0928Hyung Woo Kim

https://orcid.org/0000-0002-6305-452XChung Hee Baek

https://orcid.org/0000-0001-7611-2373Jung Tak Park

https://orcid.org/0000-0002-2325-8982Hajeong Lee

https://orcid.org/0000-0002-1873-1587Beom Jin Lim

https://orcid.org/0000-0003-2856-0133Tae-Hyun Yoo

https://orcid.org/0000-0002-9183-4507Kyung Chul Moon

https://orcid.org/0000-0002-1969-8360Ho Jun Chin

https://orcid.org/0000-0003-1185-2631Shin-Wook Kang

https://orcid.org/0000-0002-5677-4756Seung Hyeok Han

https://orcid.org/0000-0001-7923-5635 - Related articles
-
Machine learning-based 2-year risk prediction tool in immunoglobulin A nephropathy
Staphylococcal infection-associated crescentic immunoglobulin A nephropathy2017 ;36(3)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement 1
Supplement 1 Print
Print















